542865
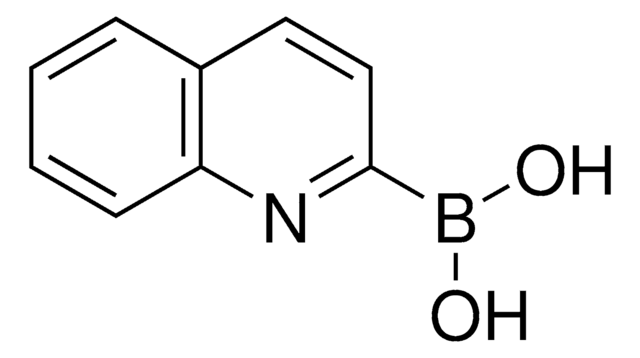
8-Quinolinylboronic acid
technical grade
Synonym(s):
8-Quinolineboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H8BNO2
CAS Number:
Molecular Weight:
172.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
form
solid
mp
>300 °C (lit.)
SMILES string
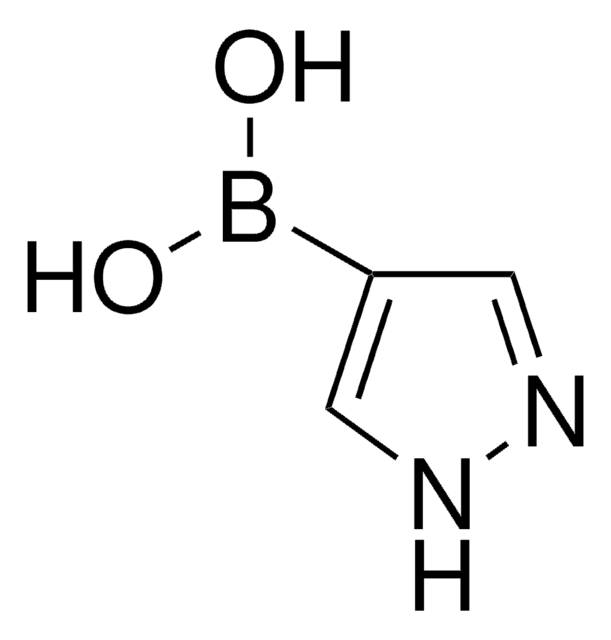
OB(O)c1cccc2cccnc12
InChI
1S/C9H8BNO2/c12-10(13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6,12-13H
InChI key
KXJJSKYICDAICD-UHFFFAOYSA-N
Application
Reactant involved in:
- C-H and C-S bond activations
- Synthesis of pyridazine via sequential amination / Suzuki coupling / alkylation reactions
- Suzuki-Miyaura coupling reactions for synthesis of biaryl monophosphorus ligands, fused tricyclic oxa-quinolones, or substituted β-amino acids
- Copper-catalyzed azidation with sodium azide
- Studies of the affect of fluoride on the stability of boronic acids during click reactions
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mayoorini Majuran et al.
ChemPlusChem, 85(2), 346-352 (2020-02-07)
We report the synthesis, photophysics, electrochemistry and electrochemiluminescence (ECL) of two dqp (dqp=2,6-di(quinoline-8-yl)pyridine) based ruthenium(II) complexes, bearing either a n-butyl ester (1) or the corresponding carboxylic acid functionality (2). The complexes were prepared from [Ru(dqp)(MeCN)3 ][PF6 ]2 by reaction with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service