All Photos(2)
About This Item
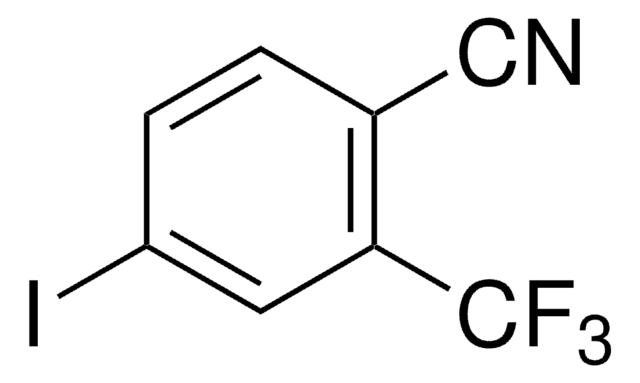
Linear Formula:
IC6H3-1,2-(CN)2
CAS Number:
Molecular Weight:
254.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
140-142 °C (lit.)
functional group
iodo
nitrile
SMILES string
Ic1ccc(C#N)c(c1)C#N
InChI
1S/C8H3IN2/c9-8-2-1-6(4-10)7(3-8)5-11/h1-3H
InChI key
OOHQHYJZUSXMFD-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Novel perfluoroalkyl phthalocyanine metal derivatives: Synthesis and photodynamic activities.
Qiu T, et al.
Dyes and Pigments, 83(1), 127-133 (2009)
Alkynyl substituted phthalocyanine derivatives as targets for optical limiting
Garcia-Frutos EM, et al.
Journal of Materials Chemistry, 13(4), 749-753 (2003)
Vibrational spectra of halophthalonitriles.
Halls MD, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 54(2), 305-317 (1998)
The synthesis of a fluorinated phthalocyanine.
Keller TM and Griffith JR.
Journal of Fluorine Chemistry, 13(1), 73-77 (1979)
Canan Uslan et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 24(2), 191-210 (2019-01-24)
Zinc(II) (5), indium(III) (6), and lutetium(III) (7) phthalocyanines (Pcs) peripherally substituted with poly (ethylene glycol) (PEG) monomethyl ether 2000 (PEGME-2000) blocks were synthesized via Sonogashira coupling reaction with high yields and their photophysical, photochemical and photobiological properties were investigated. We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service