All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H6ClN
CAS Number:
Molecular Weight:
151.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
90-95 °C/0.25 mmHg (lit.)
mp
55-58 °C (lit.)
functional group
chloro
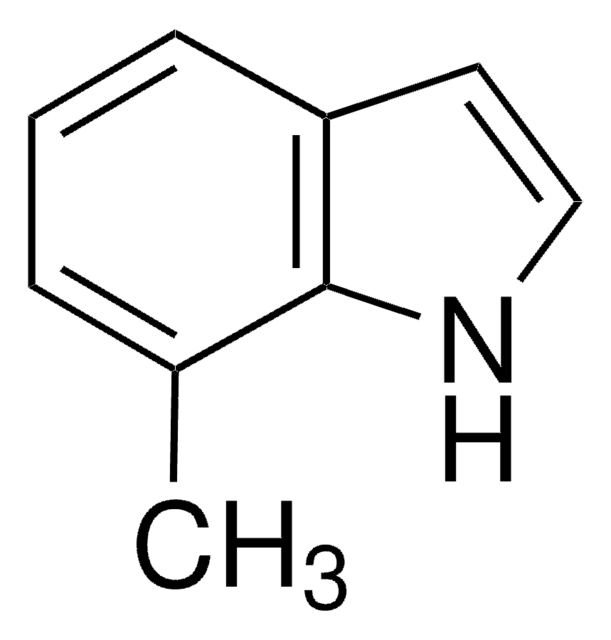
SMILES string
Clc1cccc2cc[nH]c12
InChI
1S/C8H6ClN/c9-7-3-1-2-6-4-5-10-8(6)7/h1-5,10H
InChI key
WMYQAKANKREQLM-UHFFFAOYSA-N
General description
7-Chloroindole is an indole derivative. It has been synthesized from 2,3-dihydroindole.
Application
7-Chloroindole may be used in the preparation of 1-methyl-7-chloroindole and glycosylated 7-chloroindole-3-acetamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The chemistry of indoles. XXXIX. A facile synthetic method for 7-substituted indoles.
Somei M, et al.
Chemical & Pharmaceutical Bulletin, 35(8), 3146-3154 (1987)
Xin Teng et al.
Bioorganic & medicinal chemistry letters, 15(22), 5039-5044 (2005-09-13)
Necroptosis is a regulated caspase-independent cell death mechanism that results in morphological features resembling necrosis. It can be induced in a FADD-deficient variant of human Jurkat T cells treated with TNF-alpha. 5-(1H-Indol-3-ylmethyl)-2-thiohydantoins and 5-(1H-indol-3-ylmethyl)hydantoins were found to be potent necroptosis
Synthesis of rebeccamycin and 11-dechlororebeccamycin.
Faul MM, et al.
The Journal of Organic Chemistry, 64(7), 2465-2470 (1999)
Jeongchan Lee et al.
Nature chemical biology, 17(1), 104-112 (2020-11-04)
Tyrian purple, mainly composed of 6,6'-dibromoindigo (6BrIG), is an ancient dye extracted from sea snails and was recently demonstrated as a biocompatible semiconductor material. However, its synthesis remains limited due to uncharacterized biosynthetic pathways and the difficulty of regiospecific bromination.
Chaitany Jayprakash Raorane et al.
Biomolecules, 10(8) (2020-08-23)
Multi-drug resistant Acinetobacter baumannii is well-known for its rapid acclimatization in hospital environments. The ability of the bacterium to endure desiccation and starvation on dry surfaces for up to a month results in outbreaks of health care-associated infections. Previously, indole
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service