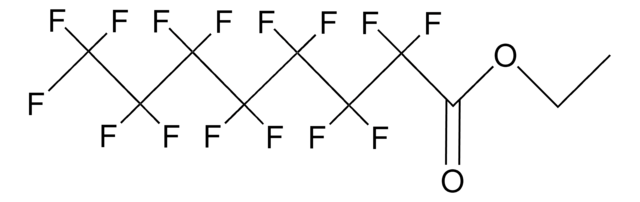
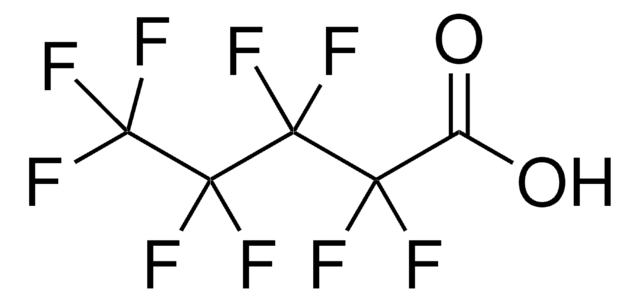
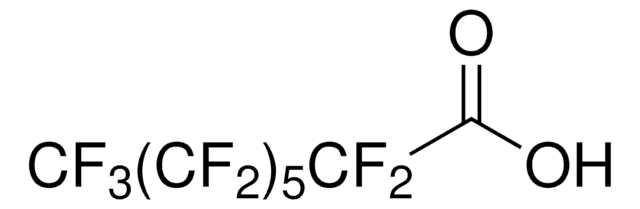406457
Methyl perfluorooctanoate
98%
Synonym(s):
Methyl pentadecafluorooctanoate
About This Item
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.305 (lit.)
bp
159-160 °C (lit.)
density
1.786 g/mL at 25 °C (lit.)
application(s)
PFAS testing
functional group
ester
fluoro
SMILES string
COC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C9H3F15O2/c1-26-2(25)3(10,11)4(12,13)5(14,15)6(16,17)7(18,19)8(20,21)9(22,23)24/h1H3
InChI key
XOCNYZFAMHDXJK-UHFFFAOYSA-N
Related Categories
General description
Application
- Synthesis of monodisperse perfluoroalkyl N-polyethoxylated amides, potential nonionic fluorinated surfactants.
- To investigate the mutual solubility curves of fluorocarbon-hydrocarbon systems.
- Synthesis of fluorine-containing monomer, N-(5-hydroxypent-1-yl)perfluorooctaneamide.
- To investigate the vinyl ether formulations for use in step and flash imprint lithography.
- Preparation of 2-chloro-1,1-dicyano-2-(perfluoroheptyl)ethylene, a precursor for synthesizing perfluorocarbon-soluble dyes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Lact. - Repr. 1B - STOT RE 1
Target Organs
Liver
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 406457-5ML | 4061836677572 |
| 406457-25ML |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service