All Photos(2)
About This Item
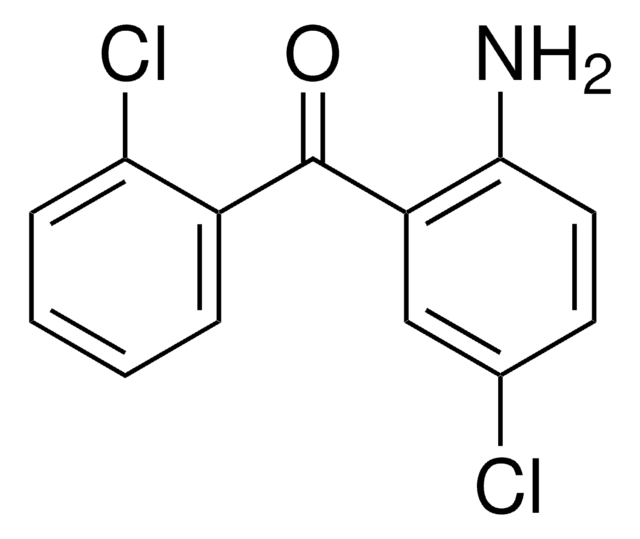
Linear Formula:
H2NC6H3(Cl)C(O)C6H4F
CAS Number:
Molecular Weight:
249.67
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
95-98 °C (lit.)
functional group
chloro
fluoro
ketone
SMILES string
Nc1ccc(Cl)cc1C(=O)c2ccccc2F
InChI
1S/C13H9ClFNO/c14-8-5-6-12(16)10(7-8)13(17)9-3-1-2-4-11(9)15/h1-7H,16H2
InChI key
GTGMXPIQRQSORU-UHFFFAOYSA-N
Application
2-Amino-5-chloro-2′-fluorobenzophenone is suitable electrophilic coupling reagent for the determination of iron(III) in environmental waters, soils and industrial effluent samples. It may be used in the synthesis of following:
- 2,4-disubstituted quinolones, via Meyer-Schuster rearrangement
- 6-chloro-2-methyl-4-(2-fluorophenyl) quinazoline
- N-desalkylflurazepam
- benzotriazepines
- diazepam-related benzodiazepines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Journal of the American Chemical Society, 112, 3969-3969 (1990)
Ionic liquid-an efficient recycalable system for the synthesis of 2, 4-disubstituted quinolines via Meyer-Schuster rearrangement.
Saqrma R and Prajapati D.
Synlett, 19, 3001-3005 (2008)
Bhawana Sati et al.
Acta pharmaceutica (Zagreb, Croatia), 63(3), 385-396 (2013-10-25)
During the manufacture of bulk drug midazolam various impurities arised that can be the related products or degradation products. Structures of eight impurities that can arise during the manufacture of bulk drug midazolam were proposed. In the present work, synthesis
J. Chem. Res. Synop., 2-2 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service