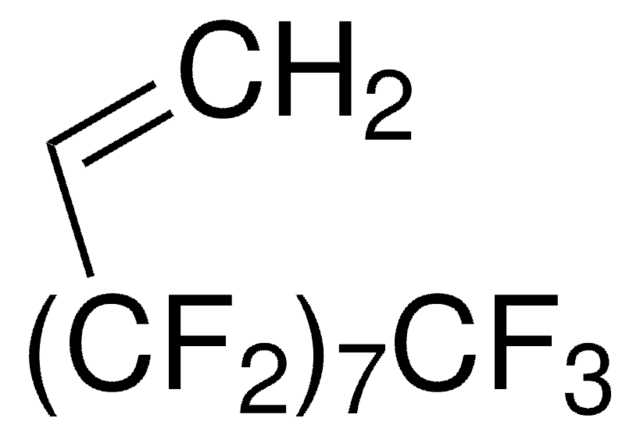371459
1H,1H,2H-Perfluoro-1-hexene
99%
Synonym(s):
3,3,4,4,5,5,6,6,6-Nonafluoro-1-hexene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
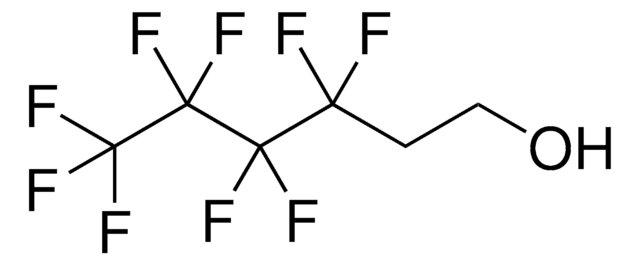
Linear Formula:
CH2=CH(CF2)3CF3
CAS Number:
Molecular Weight:
246.07
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
8.5 (vs air)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.3 (lit.)
bp
59-60 °C (lit.)
density
1.452 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C=C
InChI
1S/C6H3F9/c1-2-3(7,8)4(9,10)5(11,12)6(13,14)15/h2H,1H2
InChI key
GVEUEBXMTMZVSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Nakayama et al.
The journal of physical chemistry. A, 111(5), 909-915 (2007-02-03)
FTIR-smog chamber techniques were used to study the products of the Cl atom and OH radical initiated oxidation of CF3CH=CH2 in 700 Torr of N2/O2, diluent at 296 K. The Cl atom initiated oxidation of CF3CH=CH2 in 700 Torr of
Linda Angevine Malley et al.
Inhalation toxicology, 14(8), 773-787 (2002-07-18)
Inhalation studies were conducted to determine the potential subchronic toxicity of a mixture of trans-1,2-dichloroethylene (70%), cis-1,2-dichloroethylene (5%), and perfluorobutylethylene (25%). Groups of rats were exposed to 0, 400, 2000, or 8000 ppm concentrations of the mixture vapor 6 h/day
G L Kennedy
Critical reviews in toxicology, 21(2), 149-170 (1990-01-01)
Fluorine-containing monomers form the basis for production of a large number of commercially important polymers. Most of the polymerization occurs as gas-phase reactions, hence the hazards associated with the monomers arises primarily from inhalation. The chemicals covered in this review
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 371459-5G | |
| 371459-25G | 4061838119551 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service