302309
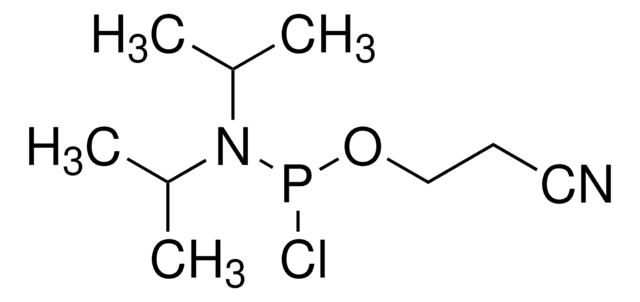
2-Cyanoethyl N,N-diisopropylchlorophosphoramidite
Cl 13.5-15.5 %
Synonym(s):
Chloro(diisopropylamino)-β-cyanoethoxyphosphine
About This Item
Recommended Products
form
liquid
composition
Cl, 13.5-15.5%
density
1.061 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
CC(C)N(C(C)C)P(Cl)OCCC#N
InChI
1S/C9H18ClN2OP/c1-8(2)12(9(3)4)14(10)13-7-5-6-11/h8-9H,5,7H2,1-4H3
InChI key
QWTBDIBOOIAZEF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- for selective monophosphorylation of carbohydrates and nucleosides
- for conversion of protected ribonucleosides to phosphoramidites
- as phosphitylating reagent for 3′-hydroxyl groups in the synthesis of oligodeoxyribonucleotides
- in a scalable, solution-phase oligonucleotide synthesis employing phosphoramidite chemistry and DMT-, iBu- and Bz-protected monomers. Intermediates were isolated by extraction without further purification
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1B
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service