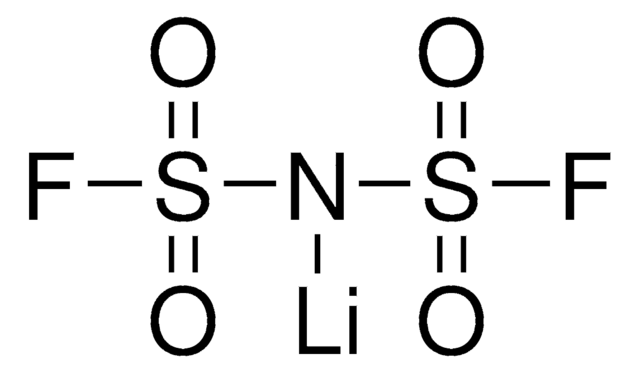282669
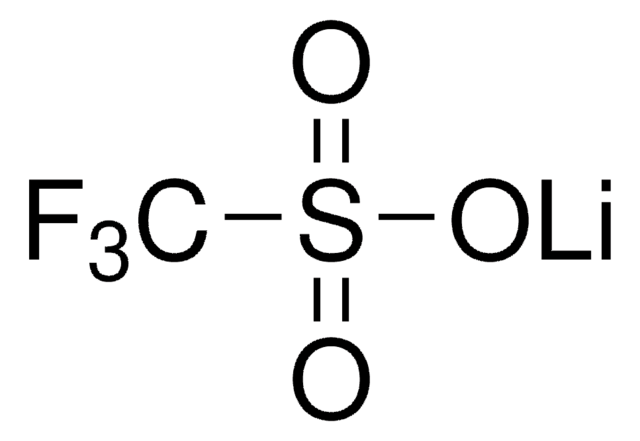
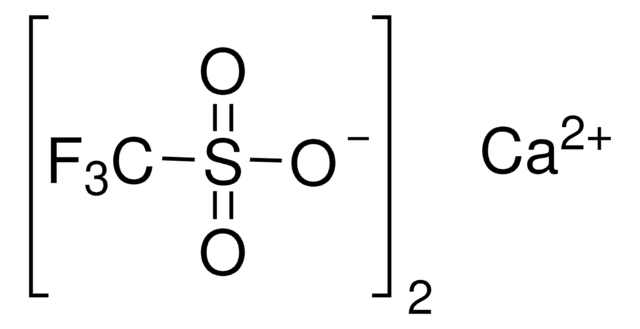
Lithium trifluoromethanesulfonate
96%
Synonym(s):
LiTf, Lithium triflate, Trifluoromethanesulfonic acid lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CF3SO3Li
CAS Number:
Molecular Weight:
156.01
Beilstein:
4301818
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
powder
mp
>300 °C (lit.)
SMILES string
[Li+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Li/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
MCVFFRWZNYZUIJ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Lithium trifluoromethanesulfonate can be used:
- As an electrolyte salt to determine the thermal stability of graphite anodes in Li-ion batteries.
- For the synthesis of solid polymer electrolytes with polyethylene oxide and chitosan, having potential applications in solid state batteries.
- As a source of triflate anion (CF3SO3−) to synthesize ionic liquids for further syntheses of conducting polymer electrolytes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
FTIR studies of chitosan acetate based polymer electrolytes.
Osman Z and Arof AK
Electrochimica Acta, 48(8), 993-999 (2003)
Ion conducting behaviour of polymer electrolytes containing ionic liquids.
Singh B and Sekhon SS
Chemical Physics Letters, 414(1-3), 34-39 (2005)
Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro-methane sulfonate (PEO-LiCF3SO3): Ionic conductivity and dielectric relaxation.
Karan NK, et al.
Solid State Ionics, 179(19-20), 689-696 (2008)
The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes.
Andersson AM, et al.
Electrochimica Acta, 47(12), 1885-1898 (2002)
Guangmin Zhou et al.
Nature communications, 6, 7760-7760 (2015-07-18)
Lithium-sulphur batteries with a high theoretical energy density are regarded as promising energy storage devices for electric vehicles and large-scale electricity storage. However, the low active material utilization, low sulphur loading and poor cycling stability restrict their practical applications. Herein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service