260843
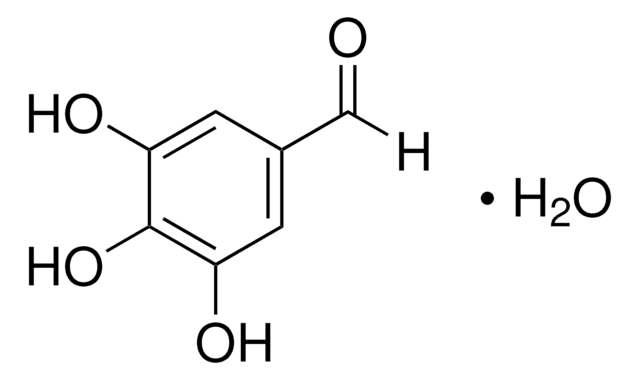
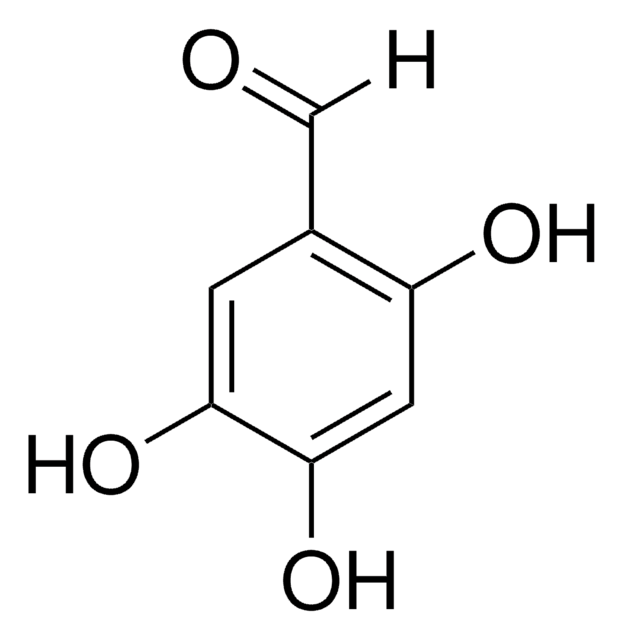
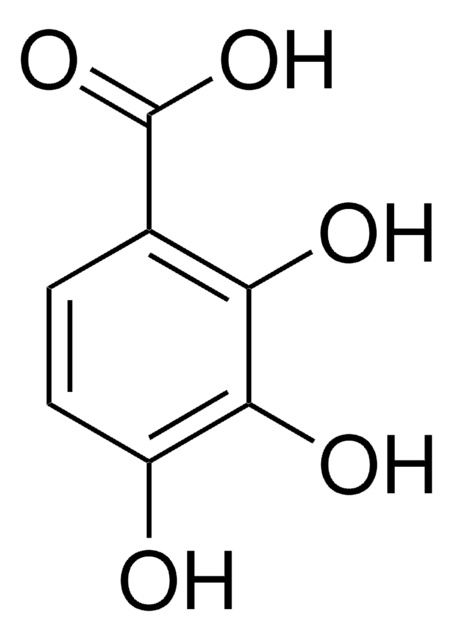
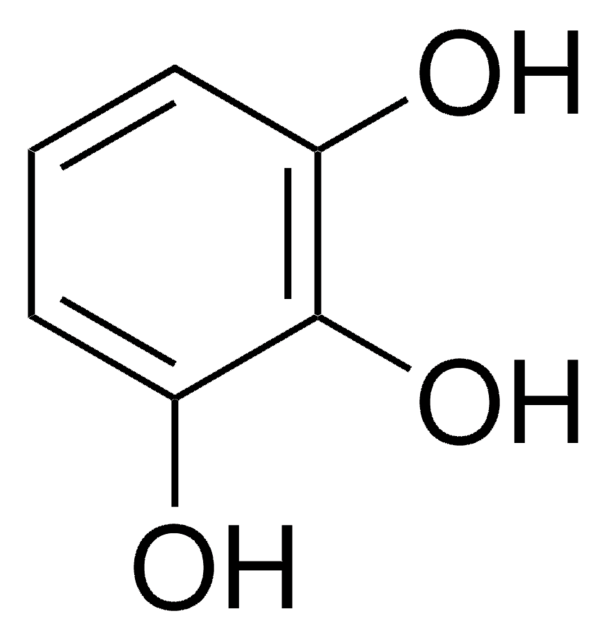
2,3,4-Trihydroxybenzaldehyde
98%
Synonym(s):
Pyrogallol-4-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)3C6H2CHO
CAS Number:
Molecular Weight:
154.12
Beilstein:
2328658
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
159-161 °C (lit.)
SMILES string
[H]C(=O)c1ccc(O)c(O)c1O
InChI
1S/C7H6O4/c8-3-4-1-2-5(9)7(11)6(4)10/h1-3,9-11H
InChI key
CRPNQSVBEWWHIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Antimicrobial activity of carbohydrazone derived from 2,3,4-trihydroxybenzaldehyde against bacteria and fungi has been investigated. 2,3,4-trihydroxybenzaldehyde forms Schiff bases via [1+1] condensation with anthranilic acid.
Application
2,3,4-Trihydroxybenzaldehyde has been used in the preparation of 1,5-dimethyl-2-phenyl-4-[(1E)-(2,3,4-trihydroxybenzylidene)amino]-1H-pyrazol-3(2H)-one.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yuan Chen et al.
International journal of biological macromolecules, 143, 714-723 (2019-11-15)
In this study, the structure of inulin was chemically modified by Schiff bases in order to improve its biological activity. A total of 6 kinds of inulin derivatives were synthesized according to aza-Wittig reaction. Their structures were confirmed by FTIR
Eila Pelttari et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 62(7-8), 483-486 (2007-10-05)
Certain substituted salicylaldehydes are known to have highly potent antimicrobial activity against bacteria and fungi, but the mechanism underlying this remarkable activity is not known, and almost nothing has been reported on the effects of further modification of the structures
B D Vázquez-Cabral et al.
Chemico-biological interactions, 272, 1-9 (2017-05-10)
Black tea infusion is the common substrate for preparing kombucha; however other sources such as oak leaves infusions can be used for the same purpose. Almost any white oak species have been used for medicinal applications by some ethnic groups
Sari Honda et al.
Free radical biology & medicine, 106, 228-235 (2017-02-23)
In this study, the mechanism of the xanthine oxidase (XO) inhibitory activity of pyrogallol, the main inhibitor found in roasted coffee, was investigated. Pyrogallol was unstable and readily converted to purpurogallin in a pH 7.4 solution, a physiological model of
1, 5-Dimethyl-2-phenyl-4-[(1E)-(2, 3, 4-trihydroxybenzylidene) amino]-1H-pyrazol-3 (2H)-one.
Sun Y-F, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(5), o2522-o2523 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service