220051
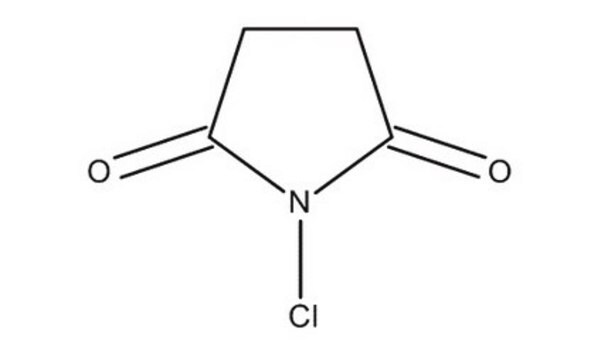
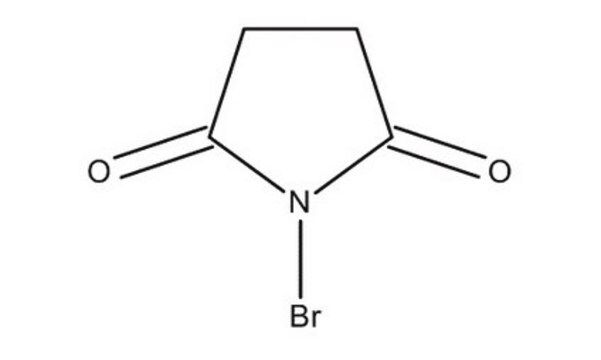
N-Iodosuccinimide
95%
Synonym(s):
1-iodo-pyrrolidine-2,5-dione, 1-iodoazolidine-2,5-dione, NIS, succiniodimide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H4INO2
CAS Number:
Molecular Weight:
224.98
Beilstein:
113917
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
mp
202-206 °C (lit.)
functional group
imide
storage temp.
2-8°C
SMILES string
IN1C(=O)CCC1=O
InChI
1S/C4H4INO2/c5-6-3(7)1-2-4(6)8/h1-2H2
InChI key
LQZMLBORDGWNPD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Highly substituted iodobenzenes prepared via an efficient 2-step process from 1,6-diynes. Used with TFA to chemoselectively hydrolyze thioglycosides to 1-hydroxyglycosides. Synthesis of vinyl sulfones from olefins and benzenesulfinic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yoshihiko Yamamoto et al.
Journal of the American Chemical Society, 128(25), 8336-8340 (2006-06-22)
Highly substituted iodobenzenes were efficiently and regioselectively synthesized from readily available 1,6-diynes via two-step process consisting of silver-catalyzed Csp-H iodination and subsequent ruthenium-catalyzed [2 + 2 + 2] cycloaddition of resultant iododiynes. Some of the obtained iodobenzenes were subjected to
Abby J Isaacs et al.
The Journal of thoracic and cardiovascular surgery, 149(5), 1262-1269 (2015-03-21)
Substantial controversy surrounds the choice between a mechanical versus bioprosthetic prosthesis for aortic valve replacement (AVR), based on age. This study aims to investigate national trends and in-hospital outcomes of the 2 prosthesis choices. All patients aged >18 years in
Canadian Journal of Chemistry, 84, 66-66 (2006)
Jasper Dinkelaar et al.
Carbohydrate research, 341(10), 1723-1729 (2006-04-06)
A variety of thioglycosides are chemoselectively hydrolyzed to the corresponding 1-hydroxy glycosides using equimolar amounts of NIS/TFA as promoter systems.
Manjunath Lamani et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(46), 14638-14642 (2012-10-09)
Single-step amination: the N-iodosuccinimide (NIS)-catalyzed amidation of acetophenone derivatives by using tert-butylhydroperoxide (TBHP) as an oxidant is presented. A variety of acetyl derivatives of heterocyclic compounds were easily converted to their corresponding ketoamides under these conditions. A new, NIS-catalyzed amination
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)