220000
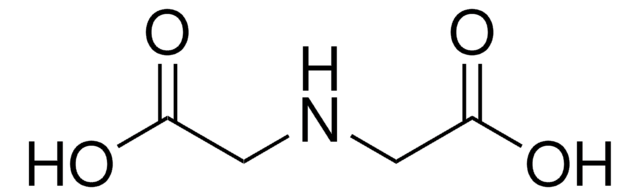
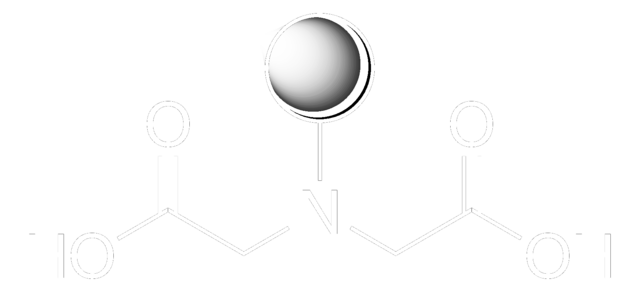
Iminodiacetic acid
98%, for peptide synthesis
Synonym(s):
2,2′-Azanediyldiacetic acid, Diglycine, IMDA
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HN(CH2COOH)2
CAS Number:
Molecular Weight:
133.10
Beilstein:
878499
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
Iminodiacetic acid, 98%
Assay
98%
form
solid
mp
243 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNCC(O)=O
InChI
1S/C4H7NO4/c6-3(7)1-5-2-4(8)9/h5H,1-2H2,(H,6,7)(H,8,9)
InChI key
NBZBKCUXIYYUSX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Iminodiacetic acid is a versatile chelating agent widely used in metal extraction and waste water treatment.
Application
Iminodiacetic acid can be used:
- In the preparation of iminodiacetic acid (IDA)-type adsorbent for protein purification.
- As a catalyst along with iron (FeCl3) complexes for methyl methacrylate (MMA) polymerization.
- In the preparation of chelating resins with glycidyl methacrylate for the selective removal and recovery of metal ions from industrial waste water.
- As a reagent for the preparation of pinene-derived iminodiacetic acid (PIDA), which is in turn used as a ligand for the synthesis of diastereoselective oxiranyl C(sp3) boronates from the corresponding olefins.
- In the surface functionalization of multi-walled carbon nanotubes (MWCNTs) which is further utilized as a sorbent for the separation of heavy metal ions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of iminodiacetic acid functionalized multi-walled carbon nanotubes and its application as sorbent for separation and preconcentration of heavy metal ions
Wang J, et al.
Journal of Hazardous Materials, 186(2-3), 1985-1992 (2011)
Chromatographic separation of proteins on metal immobilized iminodiacetic acid-bound molded monolithic rods of macroporous poly (glycidyl methacrylate-co-ethylene dimethacrylate)
Luo Q, et al.
Journal of Chromatography A, 926(2), 255-264 (2001)
AGET ATRP of methyl methacrylate catalyzed by FeCl3/iminodiacetic acid in the presence of air
Zhang L, et al.
Polymer, 49(13-14), 3054-3059 (2008)
Synthesis of chelating resins with iminodiacetic acid and its wastewater treatment application
Wang C-C, et al.
Journal of Applied Polymer Science, 84(7), 1353-1362 (2002)
Hong-Ming Yang et al.
Analytical biochemistry, 432(2), 134-138 (2012-10-03)
Highly efficient protein immobilization is extremely crucial for solid-phase immunoassays. We present a strategy for oriented immobilization of functionally intact immunoglobulin G (IgG) on a polystyrene microtiter plate via iminodiacetic acid (IDA)-Ni(2+) and ZZ-His protein interaction. We immobilized a ZZ-EAP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service