164135
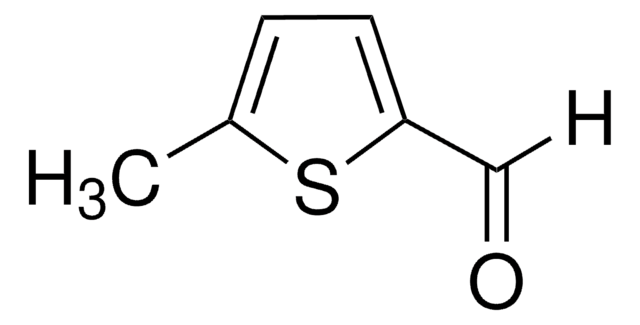
3-Methyl-2-thiophenecarboxaldehyde
90%, technical grade
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
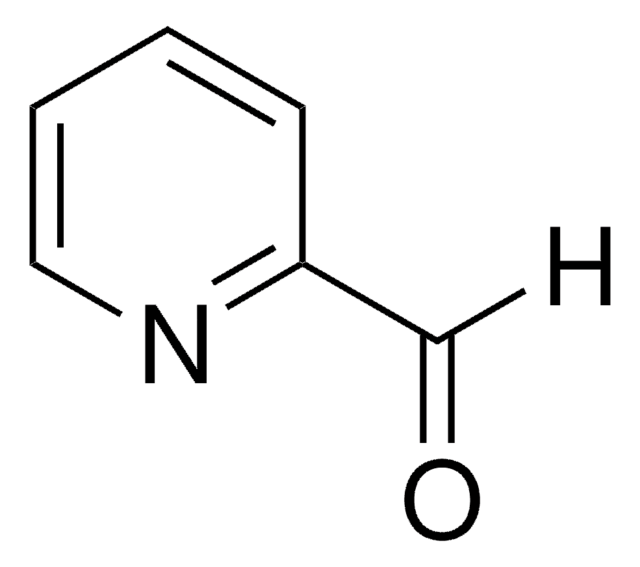
C6H6OS
CAS Number:
Molecular Weight:
126.18
Beilstein:
107874
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
refractive index
n20/D 1.587 (lit.)
density
1.17 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1sccc1C
InChI
1S/C6H6OS/c1-5-2-3-8-6(5)4-7/h2-4H,1H3
InChI key
BSQKBHXYEKVKMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Methyl-2-thiophenecarboxaldehyde was used in the synthesis of 2,3-dimethyl-5-(2,6,10-trimethylundecyl)thiophene. It was used to investigate versatile bioconversion capacity of baker′s yeast for the generation of thiols from cysteine-aldehyde conjugates.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The identification of 2, 3-dimethyl-5-(2, 6, 10-trimethylundecyl) thiophene, a novel sulphur containing biological marker.
Sinnenghe DJS, et al.
Tetrahedron Letters, 28(9), 957-960 (1987)
Tuong Huynh-Ba et al.
Journal of agricultural and food chemistry, 51(12), 3629-3635 (2003-05-29)
Baker's yeast was shown to catalyze the transformation of cysteine-furfural conjugate into 2-furfurylthiol. The biotransformation's yield and kinetics were influenced by the reaction parameters such as pH, incubation mode (aerobic and anaerobic), and substrate concentration. 2-Furfurylthiol was obtained in an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)