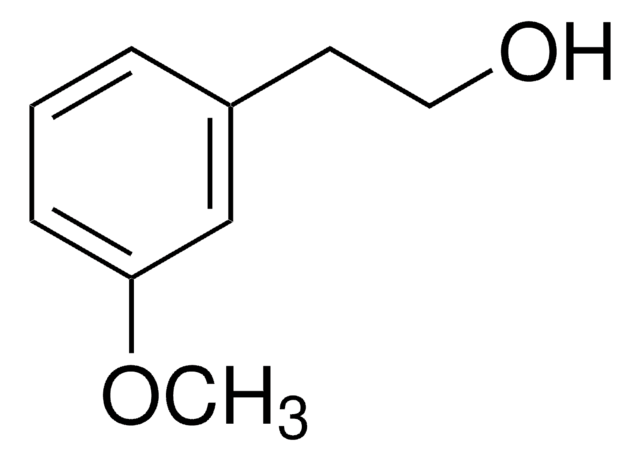
154113
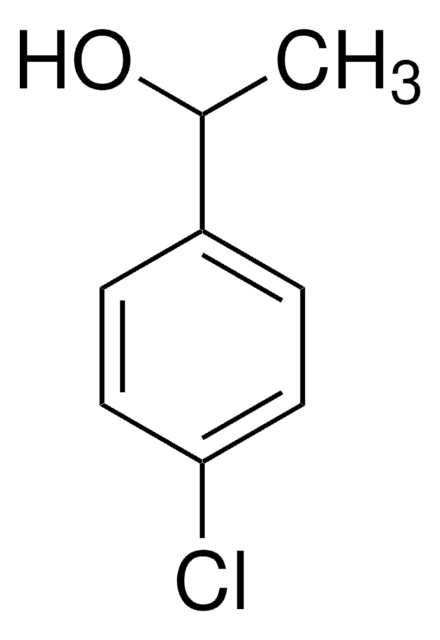
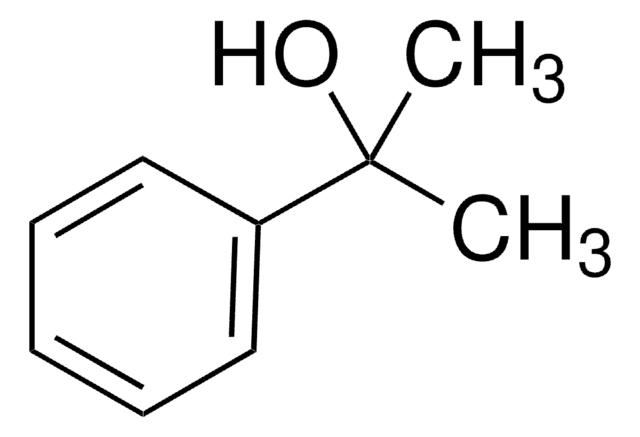
4-Methoxy-α-methylbenzyl alcohol
99%
Synonym(s):
(4-Methoxyphenyl)methylcarbinol, (±)-1-(4-Methoxyphenyl)-1-ethanol, (±)-1-(4-Methoxyphenyl)ethanol, (±)-1-(p-Methoxyphenyl)ethanol, 1-(4-Methoxyphenyl)ethan-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH(CH3)OH
CAS Number:
Molecular Weight:
152.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.533 (lit.)
bp
95 °C/1 mmHg (lit.)
density
1.079 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
COc1ccc(cc1)C(C)O
InChI
1S/C9H12O2/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7,10H,1-2H3
InChI key
IUUULXXWNYKJSL-UHFFFAOYSA-N
Related Categories
Application
4-Methoxy-α-methylbenzyl alcohol was used to study the steady-state and nanosecond, laser-flash photolysis.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tiziana Del Giacco et al.
Physical chemistry chemical physics : PCCP, 10(1), 200-210 (2007-12-14)
Steady-state and nanosecond laser flash photolysis measurements of 4-methoxybenzyl alcohol (1a), 4-methoxy-alpha-methylbenzyl alcohol (1b), 4,4'-dimethoxydiphenylmethanol (1c) and 4-methoxy-alpha,alpha'-dimethylbenzyl alcohol (1d) were carried out in air-equilibrated CH(2)Cl(2) and CH(3)CN solutions, in the presence of 9,10-dicyanoanthracene (DCA) and N-methylquinolinium tetrafluoroborate (NMQ(+)BF(4)(-)) as
Fanyu Zhang et al.
Nature communications, 11(1), 1431-1431 (2020-03-20)
The production of 2D metal-organic frameworks (MOFs) with highly exposed active surfaces is of great importance for catalysis. Here we demonstrate the formation of MOF nanosheets by utilizing CO2 as a capping agent to control the oriented growth of MOF. This
Xiao-Hong Chen et al.
PloS one, 9(4), e94543-e94543 (2014-04-18)
A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The enzyme showed a homotetrameric
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service