128910
N,O-Bis(trimethylsilyl)acetamide
synthesis grade, ≥95%
Synonym(s):
BSA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
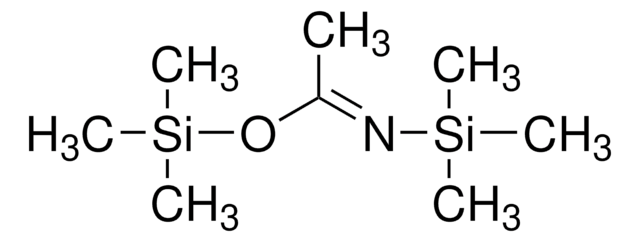
CH3C[=NSi(CH3)3]OSi(CH3)3
CAS Number:
Molecular Weight:
203.43
Beilstein:
1306669
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
synthesis grade
Quality Level
Assay
≥95%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
71-73 °C/35 mmHg (lit.)
density
0.832 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
C\C(O[Si](C)(C)C)=N/[Si](C)(C)C
InChI
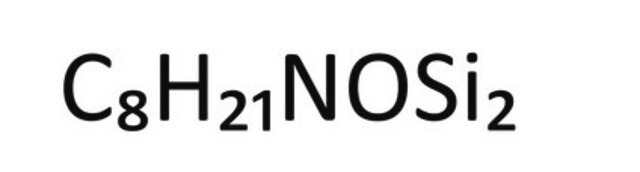
1S/C8H21NOSi2/c1-8(9-11(2,3)4)10-12(5,6)7/h1-7H3/b9-8+
InChI key
SIOVKLKJSOKLIF-CMDGGOBGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
N,O-Bis(trimethylsilyl)acetamide(BSA) is a powerful silylating agent for the protection of amides, amines, alcohols, carboxylic acids, enols and phenols. It can also be used as a Bronsted base precursor in Tsuji–Trost reactions. Additionally, it can be used to activate different functional groups during the production of nucleosides, peptides, and heterocycles.
Application
Derivatization reagent for GC-MS analysis of phenolic acids in fruits.
Reagent for the regioselective desulfation of polysaccharide sulfates. Also used to prepare trimethylsilyl ethers of carbohydrates and alcohols.
Features and Benefits
BSA is good moisture absorbent.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mohammad Saraji et al.
Journal of separation science, 29(9), 1223-1229 (2006-07-13)
A simple analytical procedure based on single-drop microextraction combined with in-syringe derivatization and GC-MS was developed for determination of some phenolic acids in fruits and fruit juices. Cinnamic acid, o-coumaric acid, caffeic acid, and p-hydroxybenzoic acid were used as model
N, O-Bis (trimethylsilyl) acetamide
Claraz A
Synlett, 24(5), 657-658 (2013)
Carbohydrate Research, 237, 313-313 (1992)
Olga Moiseeva et al.
Cell cycle (Georgetown, Tex.), 14(15), 2408-2421 (2015-06-02)
Expression of oncogenes or short telomeres can trigger an anticancer response known as cellular senescence activating the p53 and RB tumor suppressor pathways. This mechanism is switched off in most tumor cells by mutations in p53 and RB signaling pathways.
A novel regioselective desulfation of polysaccharide sulfates: Specific 6-O-desulfation with N,O-bis(trimethylsilyl)acetamide.
M Matsuo et al.
Carbohydrate research, 241, 209-215 (1993-03-17)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service