P7912
Phloretin
≥99% (HPLC), powder, GLUT inhibitor
Synonym(s):
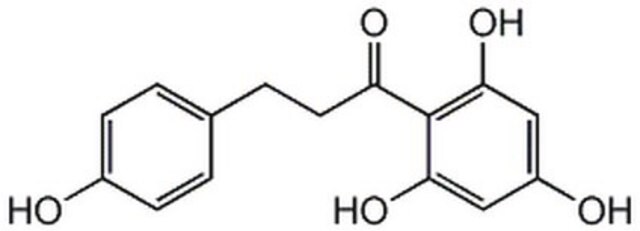
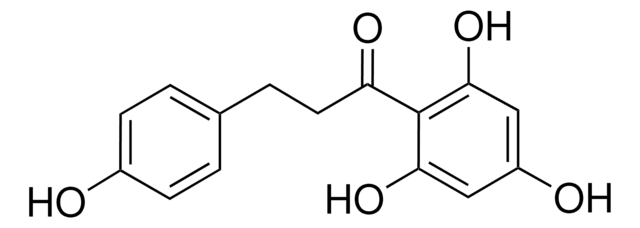
β-(4-Hydroxyphenyl)-2,4,6-trihydroxypropiophenone, 2′,4′,6′-Trihydroxy-3-(4-hydroxyphenyl)propiophenone, 3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone
About This Item
Recommended Products
product name
Phloretin, ≥99%
Quality Level
Assay
≥99%
form
powder
mp
~260 °C
storage temp.
2-8°C
SMILES string
Oc1ccc(CCC(=O)c2c(O)cc(O)cc2O)cc1
InChI
1S/C15H14O5/c16-10-4-1-9(2-5-10)3-6-12(18)15-13(19)7-11(17)8-14(15)20/h1-2,4-5,7-8,16-17,19-20H,3,6H2
InChI key
VGEREEWJJVICBM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its effect on the 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) and 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-l-glucose (2-NBDLG) uptake
- to incubate microvesicles in order to inhibit GLUT1 (glucose transporter 1)-mediated transport in radioactive ligand up-take assay
- as a component of KRH buffer to stop glucose uptake by trophoblast cells in vitro
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service