F7296
Fructosyl-Amino Acid Oxidase from Corynebacterium sp.
recombinant, expressed in E. coli, lyophilized powder, ≥0.45 units/mg protein
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
lyophilized powder
specific activity
≥0.45 units/mg protein
mol wt
~88 kDa by electrophoresis
storage temp.
−20°C
General description
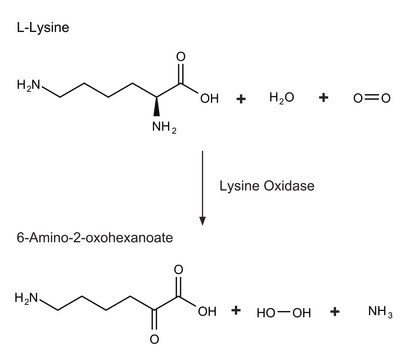
Fructosyl amino acid oxidase [fructosyl-a-l-amino acid:oxygen oxidoreductase] is a flavoprotein that catalyzes the oxidation of fructosyl amino acids to form glucosone, amino acid and hydrogen peroxide.
Enzyme Commission (E.C.) 1.5.3.x
Application
Fructosyl-Amino Acid Oxidase from Corynebacterium sp has been used in glycated haemoglobin HbA1c detection in blood samples using quartz crystal microbalance (QCM) based detection.
Fructosyl-amino acid oxidase can be used to detect the levels of glycated proteins, which are markers for diabetes mellitus.
Biochem/physiol Actions
Fructosamines are formed when glucose is condensed amino group of amino acids or proteins. Fructosamine oxidases (FAOX) catalyze the oxidative deglycation of low molecular weight fructosamines. Fructosyl amino acid oxidase catalyzes the oxidation of the C-N bond linking the C1 of the fructosyl moiety and the nitrogen of the amino group of fructosyl amino acids.
Fructosyl-Amino Acid Oxidase (FAOD) comprises of FAD-binding motifs and is classified into three types based on substrate specificity. The engineered Corynebacterium Fructosyl-Amino Acid Oxidase is stable at 45°C and could be exploited for the development of glycated protein biosensing system and glycated hemoglobin HbA1c measurements. FAOD shares sequence homology with fructosyl peptide oxidase and both are effective on α-fructosyl substrates.
Suitable for the determination of fructosyl-L-amino acid.
Unit Definition
One unit will produce 1.0 μmole of hydrogen peroxide per minute at pH 8.0 at 37 °C.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y Sakai et al.
FEBS letters, 459(2), 233-237 (1999-10-13)
A high-level production of fructosyl amino acid oxidase (FAOD), whose production was toxic in Escherichia coli, was investigated through attempts to utilize the peroxisome of Candida boidinii as the place for protein accumulation. The alcohol oxidase-depleted strain (strain aod1Delta) produced
Alteration of substrate specificity of fructosyl-amino acid oxidase from Fusarium oxysporum.
M. Fujiwara et al.
Applied Microbiology, 74, 813-819 (2007)
Ryoichi Sakaue et al.
Applied and environmental microbiology, 69(1), 139-145 (2003-01-07)
We succeeded in isolating several thermostable mutant fructosyl-amino acid oxidase (FAOX; EC 1.5.3) without reduction of productivity by directed evolution that combined an in vivo mutagenesis and membrane assay screening system. Five amino acid substitutions (T60A, A188G, M244L, N257S, and
Stefano Ferri et al.
Journal of diabetes science and technology, 3(3), 585-592 (2010-02-11)
Glycated proteins, particularly glycated hemoglobin A1c, are important markers for assessing the effectiveness of diabetes treatment. Convenient and reproducible assay systems based on the enzyme fructosyl amino acid oxidase (FAOD) have become attractive alternatives to conventional detection methods. We review
S A Schellini et al.
Acta ophthalmologica, 67(5), 601-604 (1989-10-01)
The authors report a case of fibrohistiocytoma of the limbus and discuss the clinical, histopathological and immunohistochemical findings concerning this type of lesion, with a comparison of their findings with those reported in the literature.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service