E0632
Enterokinase from porcine intestine
≥0.5 units/mg solid
Synonym(s):
Enteropeptidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
salt-free, lyophilized powder
Quality Level
specific activity
≥0.5 units/mg solid
mol wt
150 kDa
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Enterokinase from porcine intestine has been used in a study to report a new experimental model of the anomalous pancreatico-biliary junction. Enterokinase from porcine intestine has also been used in a study to investigate the insulinotropic region of the gastric inhibitory polypeptide.
The enzyme from Sigma has been used for the activation of trypsinogen in order to measure the activity of trypsin in hog pancreas. The study showed that antimicrobial treatment reduces intestinal microflora and improves protein digestive capacity without changes in villous structure of weanling pigs.
Biochem/physiol Actions
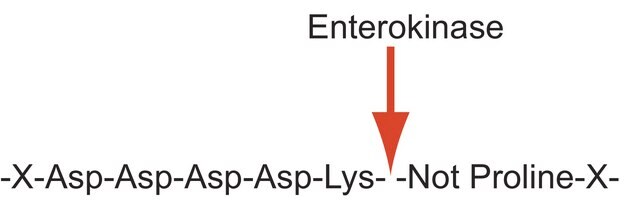
Enterokinase is a membrane bound serine protease that specifically and rapidly converts trypsinogen to trypsin, thereby, triggering the conversion of other zymogens to active enzymes. It has a molecular mass of approximately 150 kDa. The enzyme is a heterodimer consisting of 35-47 kDa subunits. The light and the heavy chains are linked by two disulfide bridges. It is a glycoprotein containing 35% carbohydrate. The polypeptide chain of trypsinogen is hydrolyzed only after an -(Asp)4-Lys- sequence. The enzyme is inhibited by soybean trypsin inhibitor. Enterokinase is typically used in protein modification and amino acid sequence determination.
Unit Definition
One unit will produce 1.0 nanomole of trypsin from trypsinogen per min at pH 5.6 at 25 °C.
substrate
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Werner Hartwig et al.
Surgery, 144(3), 394-403 (2008-08-19)
A noninvasive model of necrohemorrhagic pancreatitis induced by simultaneous intravenous cerulein/enterokinase (EK) infusion has recently been established in rats. The aim of the present study was to establish this new model in mice and to compare it with the rat
T Benhidjeb et al.
Journal of pediatric surgery, 31(12), 1670-1674 (1996-12-01)
A model of anomalous pancreatico-biliary junction was developed and used to investigate a possible role in the development of choledochal cyst and tumors of the biliary tract. An anastomosis was constructed between an isolated pancreas-duodenal segment and the gallbladder in
Thomas Thymann et al.
The British journal of nutrition, 97(6), 1128-1137 (2007-03-27)
The immediate post-weaning period is often associated with gut malfunction and diarrhoea for young pigs. Administration of antimicrobials remains an effective way to control weaning diarrhoea but it remains unclear how they affect gut physiology and microbiology although this is
G W Morrow et al.
Canadian journal of physiology and pharmacology, 74(1), 65-72 (1996-01-01)
Glucose-dependent insulinotropic polypeptide or gastric inhibitory polypeptide (GIP) is a 42 amino acid intestinal hormone, which exhibits several direct and indirect effects on fat and glucose metabolism. To determine the bioactive region(s) of the molecule, synthetic and proteolytic fragments of
Complementary DNA cloning and sequencing of rat enteropeptidase and tissue distribution of its mRNA.
N Yahagi et al.
Biochemical and biophysical research communications, 219(3), 806-812 (1996-02-27)
A cDNA clone encoding enteropeptidase (EC 3.4.21.9), a key enzyme for the conversion of trypsinogen to trypsin, was isolated from a rat duodenal mucosa cDNA library. Sequences of the 3585 base pair clone predicted that enteropeptidase is synthesized as a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service