D193
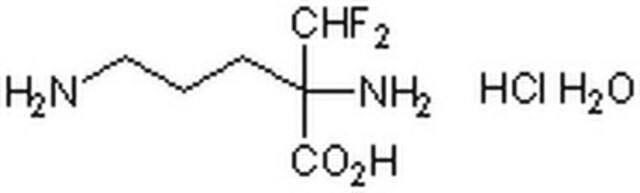
DL-α-Difluoromethylornithine hydrochloride hydrate
≥97% (NMR), solid, polyamine biosynthesis inhibitor
Synonym(s):
2-(Difluoromethyl)ornithine hydrochloride hydrate, DFMO hydrochloride hydrate, Eflornithine hydrochloride hydrate
About This Item
Recommended Products
Product Name
DL-α-Difluoromethylornithine hydrochloride hydrate, solid, ≥97% (NMR)
Quality Level
Assay
≥97% (NMR)
form
solid
color
white
solubility
DMSO: >10 mg/mL
H2O: >10 mg/mL
SMILES string
O.Cl.NCCCC(N)(C(F)F)C(O)=O
InChI
1S/C6H12F2N2O2.ClH.H2O/c7-4(8)6(10,5(11)12)2-1-3-9;;/h4H,1-3,9-10H2,(H,11,12);1H;1H2
InChI key
FJPAMFNRCFEGSD-UHFFFAOYSA-N
Gene Information
human ... ODC1(4953)
General description
Application
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service