C4290
Butyrylcholinesterase from equine serum
lyophilized powder, ≥500 units/mg protein
Synonym(s):
Acylcholine acyl-hydrolase, Choline esterase, butyryl, Pseudocholinesterase
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Recommended Products
biological source
equine serum
Quality Level
form
lyophilized powder
specific activity
≥500 units/mg protein
composition
Protein, ≥10%
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Butyrylcholinesterase (BChE) from equine serum has been used:
- to determine the inhibitory concentration of bupivacaine on butyrylcholinesterase
- in acetylcholinesterase (AChE)/BChE activity assay to determine the inhibitory activity of benzothiazole-piperazine compounds
Selective inhibition of BChE activity can be used in the detection of organophosphates. Its use in the treatment of organophosphate toxicity shows promise. There is a correlation between the level of BChE in human blood and degree of protection against potentially toxic nerve agents. There has also been interest in the roles of cholinesterases with regard to Alzheimer′s disease. Investigations into selective inhibitors may provide a clearer picture of the physiological role of BChE in both healthy and diseased individuals. The enzyme has been used to test bupivacaine as an inhibitor of butyrylcholinesterase during acetylcholinesterase assay using cerebrospinal fluid.
Biochem/physiol Actions
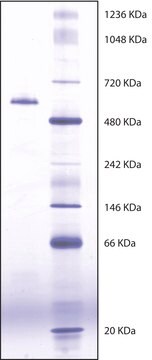
Butyrylcholinesterase (BChE) is a serine hydrolase that is structurally similar to acetylcholinesterase (AChE), but differs in substrate specificities and inhibitor sensitivities. BChE can, unlike AChE, efficiently hydrolyze larger esters of choline such as butyrylcholine and benzoylcholine. The enzyme is a tetrameric glycoprotein with four equal subunits (110 kDa each). The enzyme is activated by Ca2+ and Mg2+ and the activity is constant over the pH range 6.0-8.0. It is inhibited by Betaine, nicotine, organophosphates, carbamates.
Unit Definition
One unit will hydrolyze 1.0 μmole of butyrylcholine to choline and butyrate per min at pH 8.0 at 37 °C. The activity obtained using butyrylcholine as substrate is ~2.5 times that obtained using acetylcholine.
Physical form
Highly purified, lyophilized powder containing buffer salts
Analysis Note
Protein determined by biuret
inhibitor
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of thyroxine binding globulin.
Bergmeyer, H.U
Methods of Enzymatic Analysis, 2, 833-833 (1974)
W H Kluge et al.
BMC biochemistry, 2, 17-17 (2002-01-22)
Most test systems for acetylcholinesterase activity (E.C.3.1.1.7.) are using toxic inhibitors (BW284c51 and iso-OMPA) to distinguish the enzyme from butyrylcholinesterase (E.C.3.1.1.8.) which occurs simultaneously in the cerebrospinal fluid. Applying Ellman's colorimetric method, we were looking for a non-toxic inhibitor to
Physical properties and subunit structure of butyrylcholinesterase from horse serum.
J C Lee et al.
Biochemistry, 12(8), 1622-1630 (1973-04-10)
Acetylcholinesterase assay for cerebrospinal fluid using bupivacaine to inhibit butyrylcholinesterase
Kluge WH, et al.
BMC Biochemistry, 2(1), 17-17 (2001)
Luisa Savini et al.
Journal of medicinal chemistry, 46(1), 1-4 (2002-12-28)
Tacrine-based AChE and BuChE inhibitors were designed by investigating the topology of the active site gorge of the two enzymes. The homobivalent ligands characterized by a nitrogen-bridged atom at the tether level could be considered among the most potent and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service