14701C
EX-CELL® Glycosylation Adjust (Gal +)
protein quality supplement
Synonym(s):
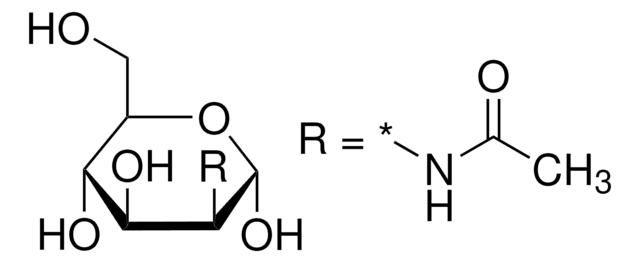
N-linked glycosylation reagent
About This Item
Recommended Products
form
liquid
quality
GMP
chemically defined
technique(s)
cell culture | mammalian: suitable
impurities
Sterility Tested, pH, Endotoxin, and Appearance
suitability
suitable for manufacturing use
storage temp.
2-8°C
General description
Packaging
14701C-1000ML
Linkage
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Our protein quality supplement, EX-CELL® Glycosylation Adjust (Gal+), provides customers with a novel chemically defined product which targets glycosylation attributes.
The biosimilar market is expected to grow rapidly in the coming years, as many blockbuster monoclonal antibodies lose patent protection by 2020.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service