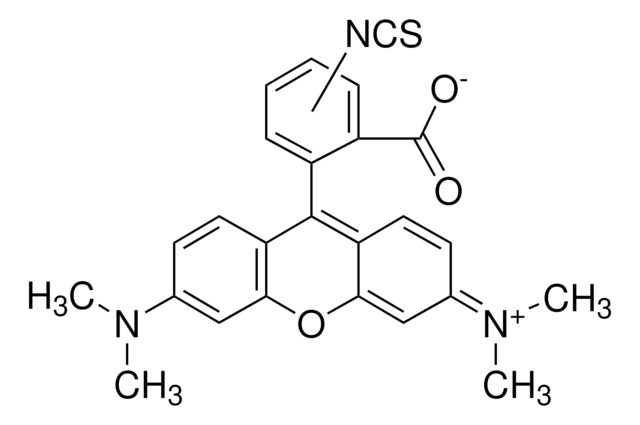
T3163
Tetramethylrhodamine isothiocyanate Isomer R
powder
Synonym(s):
6-TRITC, Tetramethylrhodamine-6-isothiocyanate
About This Item
Recommended Products
description
labeling efficiency with bovine albumin >= 50%
Quality Level
form
powder
composition
Dye content, ≥80%
solubility
1 M NH4OH: 10 mg/mL (Faint Red to Very Dark Red to Very Dark Purple solution)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
2-8°C
SMILES string
CN(C)c1ccc2c(OC3=C\C(C=CC3=C2c4cc(ccc4C([O-])=O)N=C=S)=[N+](/C)C)c1
InChI
1S/C25H21N3O3S/c1-27(2)16-6-9-19-22(12-16)31-23-13-17(28(3)4)7-10-20(23)24(19)21-11-15(26-14-32)5-8-18(21)25(29)30/h5-13H,1-4H3
InChI key
OBYNJKLOYWCXEP-UHFFFAOYSA-N
General description
Application
- TRITC is used to label a wide variety of biomolecules, including immunoglobulins, lectins, nucleic acids, polynucleotides, and polysaccharides for affinity, immuno-, and in situ hybridization, fluorescent probes, and flow cytometry.
- Lectins are TRITC-Iabeled reagents for the affinity staining of sections and cell monolayers to distinguish the sialomucins of salivary glands.
- TRITC-antibodies are used to identify pathogenic amoebae.
- TRITC-oligonucleotides are used in the in-situ hybridization staining of soil microorganisms.
- TRITC-Iabeled reagents have also been used as fluorescent probes of live cells.
- Flow cytometry of TRlTC-Iabeled slime mold cells has been used to study their aggregation behavior.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Import Using Xenopus Egg Extracts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service