PHR1669
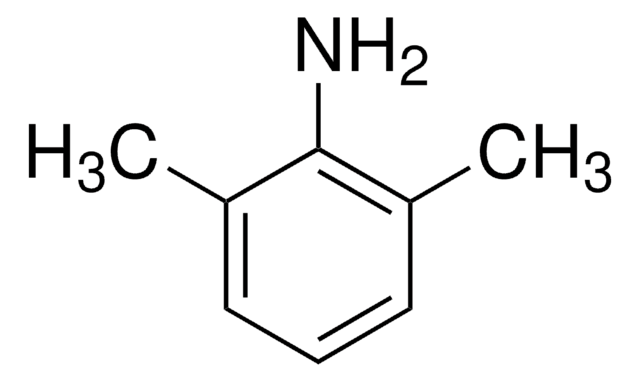
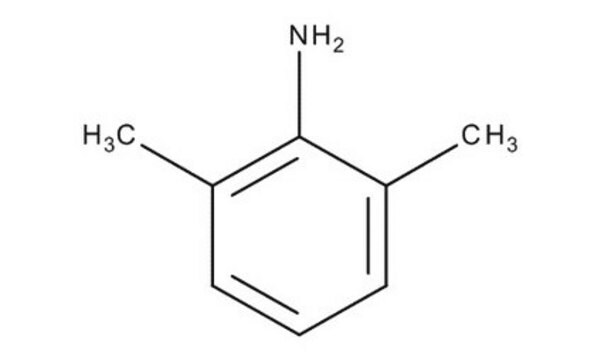
Lidocaine Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(s):
2,6-Dimethylaniline, Lidocaine Impurity A; 2,6 DMA, 2,6-Xylidine, 2-Amino-1,3-dimethylbenzene, 2-Amino-m-xylene
About This Item
Recommended Products
grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. Y0001575
vapor pressure
<0.01 mmHg ( 20 °C)
API family
lidocaine
CofA
current certificate can be downloaded
packaging
pkg of 100 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.560 (lit.)
bp
214 °C/739 mmHg (lit.)
mp
10-12 °C (lit.)
density
0.984 g/mL at 25 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
SMILES string
Cc1cccc(C)c1N
InChI
1S/C8H11N/c1-6-4-3-5-7(2)8(6)9/h3-5H,9H2,1-2H3
InChI key
UFFBMTHBGFGIHF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The standard is a certified reference material (CRM) qualified with instruments validated according to good manufacturing practices (GMP) using pharmacopeia monograph methods. It is supplied with a comprehensive certificate containing information on traceability assay results, certified purity, homogeneity tests, uncertainty statement, and stability assessment.
Lidocaine Related Compound A is a primary aromatic amine and a major metabolite of the anesthetic lidocaine. It is used as a starting material in the manufacturing of various anesthetics like lidocaine, bupivacaine, mepivacaine, etidocaine, ropivacaine, pyrrocaine, and xylazine.
Application
This pharmaceutical secondary standard can also be used as follows:
- Development of an impurity selective reverse phase-high performance liquid chromatography (RP-HPLC) method to determine dexpanthenol, lidocaine hydrochloride, mepyramine maleate, and their related substances in topical dosage forms
- Testing a selective high-performance liquid chromatography-diode array detection (HPLC-DAD) method, developed for the simultaneous analysis of miconazole nitrate and lidocaine hydrochloride in their combined oral gel dosage form, for its stability-indicating properties
- Evaluation of a high-performance liquid chromatography-diode array detection (HPLC-DAD) procedure― for its stability indicating properties, developed to determine nitrofurazone and lidocaine hydrochloride in their combined dosage form
- Separation of 2,6-Dimethylaniline, its isomeric impurities, and other related impurities by isocratic and reverse-phase ultra-performance liquid chromatographic (UPLC) method
- analyze a binary mixture of lidocaine hydrochloride and cetylpyridinium chloride in presence of lidocaine impurity A by spectrophotometric methods
- determine lidocaine hydrochloride-related substance by analytical methods in pharmaceutical dosage forms
Analysis Note
Footnote
Recommended products
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
195.8 °F - closed cup
Flash Point(C)
91 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
GC Analysis of Anilines on Equity®-5
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service