69484
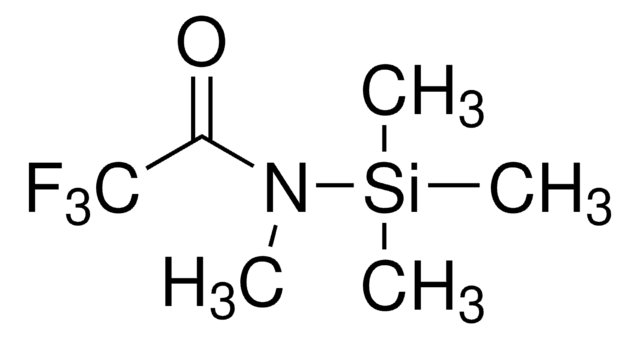
N-Methyl-N-trimethylsilylheptafluorobutyramide
for GC derivatization, LiChropur™, ≥90% (GC)
Synonym(s):
N-Trimethylsilyl-N-methylheptafluorobutyramide, MSHFBA
About This Item
Recommended Products
grade
for GC derivatization
Quality Level
Assay
≥90% (GC)
form
liquid
quality
LiChropur™
reaction suitability
reagent type: derivatization reagent
reaction type: Silylations
technique(s)
gas chromatography (GC): suitable
refractive index
n20/D 1.353 (lit.)
n20/D 1.353
bp
148 °C (lit.)
density
1.254 g/mL at 25 °C (lit.)
SMILES string
CN(C(=O)C(F)(F)C(F)(F)C(F)(F)F)[Si](C)(C)C
InChI
1S/C8H12F7NOSi/c1-16(18(2,3)4)5(17)6(9,10)7(11,12)8(13,14)15/h1-4H3
InChI key
CMXKINNDZCNCEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Simultaneous quantitation of cocaine, opiates, and their metabolites in human hair by positive ion chemical ionization gas chromatography-mass spectrometry: This study demonstrates the application of N-Methyl-N-trimethylsilylheptafluorobutyramide in forensic toxicology to analyze drug residues in human hair, providing a robust method for detecting such compounds at trace levels (Höld KM, Wilkins DG, Rollins DE, Joseph RE Jr, Cone EJ, 1998).
- Detection of stanozolol in hair by negative ion chemical ionization mass spectrometry: The research utilizes N-Methyl-N-trimethylsilylheptafluorobutyramide for the sensitive detection of stanozolol, a performance-enhancing steroid, in hair samples. This method is particularly useful in sports doping analyses to ensure fair play (Höld KM, Wilkins DG, Crouch DJ, Rollins DE, Maes RA, 1996).
Other Notes
Legal Information
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service