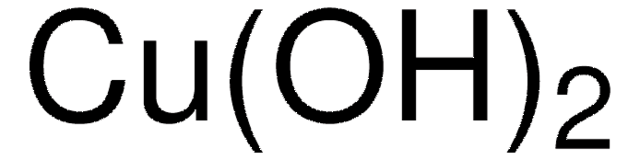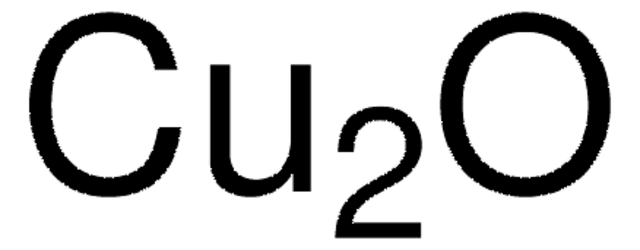566284
Copper(I) oxide
≥99.99% trace metals basis, anhydrous
Synonym(s):
Cuprous oxide
About This Item
Recommended Products
grade
anhydrous
Quality Level
Assay
≥99.99% trace metals basis
form
powder
density
6 g/mL at 25 °C (lit.)
SMILES string
[Cu]O[Cu]
InChI
1S/2Cu.O
InChI key
BERDEBHAJNAUOM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a precursor to prepare nanoparticles, nanocrystals, and quantum dots for various applications.
- To catalyze the N-arylation of azoles under aqueous conditions using phase-transfer catalyst.
- To prepare reduced graphene oxide-based nano copper composites applicable as a sensor for the detection of dopamine.
- As a copper precursor to prepare Cu2O-TiO2 nanoparticle-based composite materials with aluminosilicate geopolymers applicable in the removal of organic pollutants from water.
accessory
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nanostructured Materials Through Ultrasonic Spray Pyrolysis
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service