ECM210
QCM Endothelial Cell Invasion Assay (24 well, colorimetric)
This QCM Endothelial Cell Invasion Assay provides an in vitro model to quickly screen factors that can regulate endothelial invasion. The assay is performed in an invasion chamber using a basement membrane protein coated on the porous insert.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352207
eCl@ss:
32161000
NACRES:
NA.84
Recommended Products
Quality Level
manufacturer/tradename
Chemicon®
QCM
technique(s)
cell based assay: suitable
detection method
colorimetric
shipped in
wet ice
General description
Also available: Cell Comb Scratch Assay! Get biochemical data from a scratch assay!Click Here
Introduction
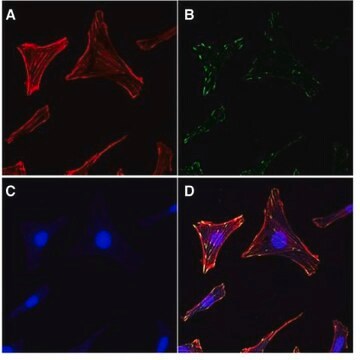
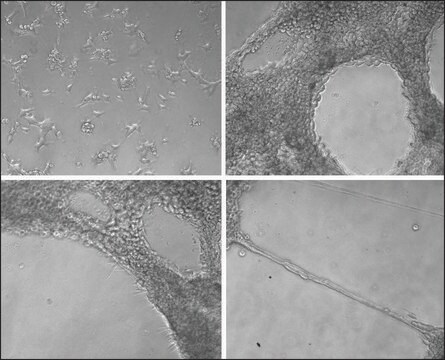
Endothelial cells (EC) invade through the basement membrane (BM) to form sprouting vessels. The invasion process consists of the secretion of matrix metalloproteases (MMP) to degrade basement membrane, the activation of endothelial cells, and the migration of EC across the basement membrane. The understanding of EC invasion is important for studying the mechanism of angiogenesis in injured tissue as well as in disease such as cancer.
Cell migration may be evaluated through several different methods, the most widely accepted of which is the Boyden Chamber assay. The Boyden Chamber system uses two-chamber system which a porous membrane provides an interface between two chambers. Cells are seeded in the upper chamber and chemoattractants placed in the lower chamber. Cells in the upper chamber migrate toward the chemoattractants by passing through the porous membrane to the lower chamber. Migratory cells are then stained and quantified.
Introduction
Endothelial cells (EC) invade through the basement membrane (BM) to form sprouting vessels. The invasion process consists of the secretion of matrix metalloproteases (MMP) to degrade basement membrane, the activation of endothelial cells, and the migration of EC across the basement membrane. The understanding of EC invasion is important for studying the mechanism of angiogenesis in injured tissue as well as in disease such as cancer.
Cell migration may be evaluated through several different methods, the most widely accepted of which is the Boyden Chamber assay. The Boyden Chamber system uses two-chamber system which a porous membrane provides an interface between two chambers. Cells are seeded in the upper chamber and chemoattractants placed in the lower chamber. Cells in the upper chamber migrate toward the chemoattractants by passing through the porous membrane to the lower chamber. Migratory cells are then stained and quantified.
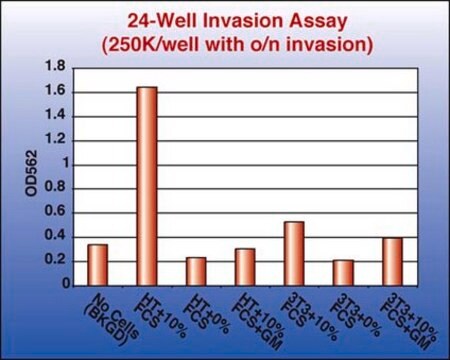
Application
Millipore’s QCM Endothelial Cell Invasion Assay provides an in vitro model to quickly screen factors that can regulate endothelial invasion. The assay is performed in an invasion chamber using a basement membrane protein coated on the porous insert. The level of coating and the pore size is optimized for endothelial cells so the researcher may utilize this kit to mimic physiological condition. After cell invasion has occurred, the researcher can select a staining method to quantify the number of cells that have invaded through the chamber. Millipore offers a colorimetric staining kit (ECM210) and a fluorometric kit staining reagent (ECM211) for both convenience and efficiency.
Research Category
Apoptosis & Cancer
Cell Structure
Apoptosis & Cancer
Cell Structure
Packaging
Sufficient for 24 assays
Components
1. Cell Invasion Plate Assembly: (Part No. CS203020) Two 24-well plates each containing 12 ECMatrix-coated 3 m inserts per plate.
2. Cell Stain Solution: (Part No. 90144)* One bottle.
3. Extraction Buffer: (Part No. 90145) One bottle.
4. 24-well Stain Extraction Plate: (Part No. 2005871) One each.
4. 24-well Stain Extraction Plate: (Part No. 2005871) One each.
5. 96-well Stain Quantitation Plate: (Part No. 2005870) One each.
6. Cotton Swabs: (Part No. 10202) Fifty each.
7. Forceps: (Part No. 10203) One each.
2. Cell Stain Solution: (Part No. 90144)* One bottle.
3. Extraction Buffer: (Part No. 90145) One bottle.
4. 24-well Stain Extraction Plate: (Part No. 2005871) One each.
4. 24-well Stain Extraction Plate: (Part No. 2005871) One each.
5. 96-well Stain Quantitation Plate: (Part No. 2005870) One each.
6. Cotton Swabs: (Part No. 10202) Fifty each.
7. Forceps: (Part No. 10203) One each.
Storage and Stability
Store kit materials at 2° to 8°C for up to their expiration date. Do not freeze.
Legal Information
Accutase is a registered trademark of Innovative Cell Technologies, Inc.
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
Flash Point(F)
53.6 °F
Flash Point(C)
12 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cell based angiogenesis assays to analyze new blood vessel formation for applications of cancer research, tissue regeneration and vascular biology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service