208743
Calpain Inhibitor XI
The Calpain Inhibitor XI, also referenced under CAS 145731-49-3, controls the biological activity of Calpain. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
Synonym(s):
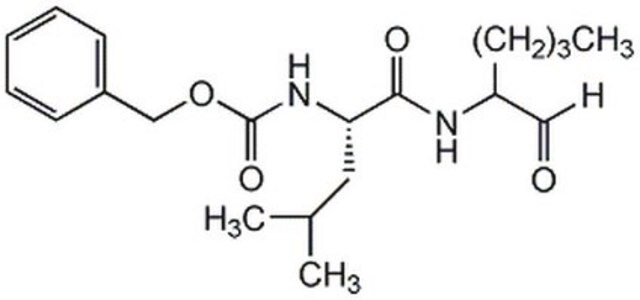
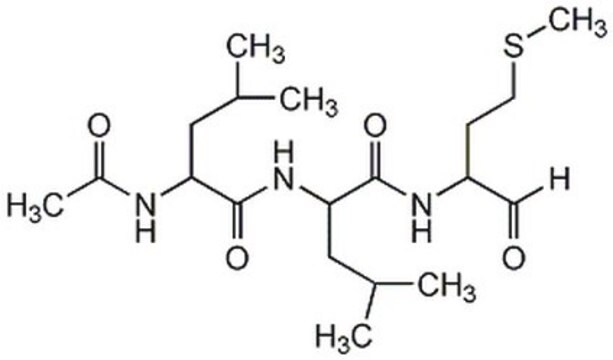
Calpain Inhibitor XI, Z-L-Abu-CONH(CH₂)₃-morpholine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H40N4O6
CAS Number:
Molecular Weight:
504.62
UNSPSC Code:
12352200
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
color
white to off-white
solubility
DMSO: 5 mg/mL
shipped in
ambient
storage temp.
2-8°C
General description
A cell-permeable dipeptidyl α-ketoamide that acts as a potent, highly selective, reversible, and active site inhibitor of calpain-1 and -2 (Ki = 140 nM and 41 nM, respectively). Weakly inhibits cathepsin B (Ki = 6.9 µM). Reported to have a neuroprotective role in the central nervous system following focal ischemia. Also protects against virus-induced apoptotic myocardial injury in mice.
A cell-permeable dipeptidyl a-ketoamide that acts as a potent, highly selective, reversible, active site inhibitor of calpain-1 (Ki = 140 nM) and calpain-2 (Ki = 41 nM). Weakly inhibits cathepsin B (Ki = 6.9 µM). Shown to inhibit calpain-m-mediated degradation of neurofilament protein (NFP) (IC50 = 600 nM). Also shown to exhibit neuroprotective effects in the central nervous system following focal ischemia. Reported to protect against reovirus-induced myocarditis in mice.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
calpain 1, calpain 2
calpain 1, calpain 2
Product does not compete with ATP.
Reversible: yes
Target Ki: 140 nM and 41 nM, against calpain-1 and -2, respectively
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Sequence
Z-Leu-Abu-CONH(CH₂)₃-morpholine (Abu = α-aminobutyric acid)
Physical form
Supplied as a trifluoroacetate salt.
Reconstitution
Following reconstitution aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Other Notes
Blomgren, K., et al. 2001. J. Biol. Chem.276, 10191.
DeBiasi, R.L., et al. 2001. J. Virol.75, 351.
Saatman, K.E., et al. 2000. J. Cereb. Blood Flow Metab.20, 66.
Stelmasiak, Z., et al. 2000. Med. Sci. Monit.6, 426.
Blomgren, K., et al. 1999. J. Biol. Chem.274, 14046.
James, T., et al. 1998. J. Neurosci. Res.51, 218.
Li, Z., et al. 1996. J. Med. Chem.39, 4089.
Saatman, K.E, et al. 1996. Proc. Natl. Acad. Sci. USA93, 3428.
Bartus, R.T., et al. 1995. Neurol. Res.17, 249.
Bartus, R.T., et al. 1994. Stroke25, 2265.
DeBiasi, R.L., et al. 2001. J. Virol.75, 351.
Saatman, K.E., et al. 2000. J. Cereb. Blood Flow Metab.20, 66.
Stelmasiak, Z., et al. 2000. Med. Sci. Monit.6, 426.
Blomgren, K., et al. 1999. J. Biol. Chem.274, 14046.
James, T., et al. 1998. J. Neurosci. Res.51, 218.
Li, Z., et al. 1996. J. Med. Chem.39, 4089.
Saatman, K.E, et al. 1996. Proc. Natl. Acad. Sci. USA93, 3428.
Bartus, R.T., et al. 1995. Neurol. Res.17, 249.
Bartus, R.T., et al. 1994. Stroke25, 2265.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service