E50604
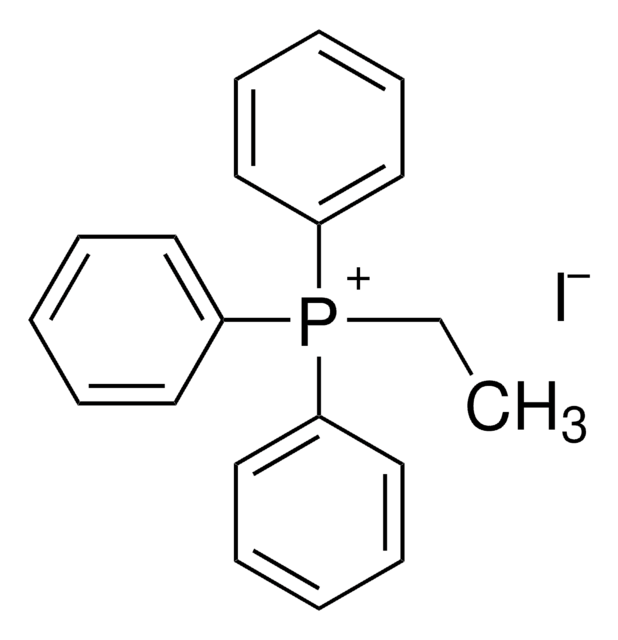
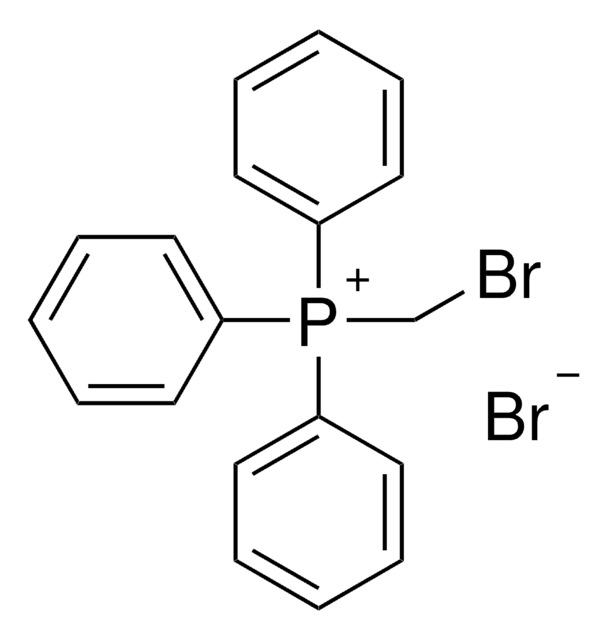
Ethyltriphenylphosphonium bromide
99%
Synonym(s):
TEP
About This Item
Recommended Products
Quality Level
Assay
99%
reaction suitability
reaction type: C-C Bond Formation
mp
203-205 °C (lit.)
SMILES string
[Br-].CC[P+](c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C20H20P.BrH/c1-2-21(18-12-6-3-7-13-18,19-14-8-4-9-15-19)20-16-10-5-11-17-20;/h3-17H,2H2,1H3;1H/q+1;/p-1
InChI key
JHYNXXDQQHTCHJ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- D-amino acids from L-cysteine-derived thiazolidines
- Leiodolide A via aldol reactions and Horner-Wadsworth-Emmons olefination
- Cycloalkanoindolines via diastereoselective intramolecular inimo-ene reactions
- WXYZA′ domain of maitotoxin using the coupling of key building blocks
Reactant for:
- Solid-state metathesis polycondensation to form alkyl-dipropenylthiophene monomers
- Mizoroki-Heck cyclization and cascading Tsuji-Trost cyclization / lactonization cyclization reactions for synthesis of an ABC ring system
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 2
Flash Point(F)
401.0 °F
Flash Point(C)
205 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| E50604-100G | 4061833604144 |
| E50604-25G | 4061833604151 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service