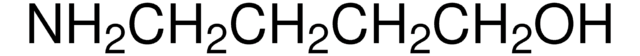A56353
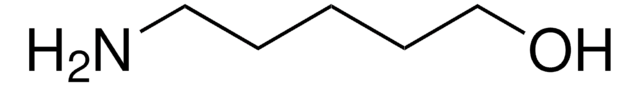
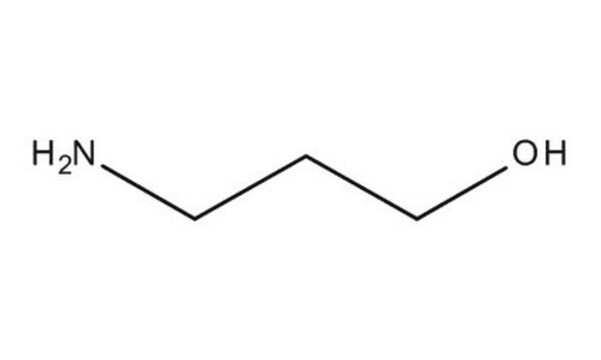
6-Amino-1-hexanol
97%
Synonym(s):
1-Amino-6-hexanol, 1-Amino-6-hydroxyhexane, 6-Amino-1-hexanol, 6-Hexanolamine, 6-Hydroxy-1-hexylamine, 6-Hydroxyhexylamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2N(CH2)6OH
CAS Number:
Molecular Weight:
117.19
Beilstein:
1732524
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
bp
135-140 °C/30 mmHg (lit.)
mp
54-58 °C (lit.)
SMILES string
NCCCCCCO
InChI
1S/C6H15NO/c7-5-3-1-2-4-6-8/h8H,1-7H2
InChI key
SUTWPJHCRAITLU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Amino-1-hexanol can undergo cyclization over copper supported on γ-alumina and magnesia to form hexahydro-1H-azepine. It may be used along with glutaric acid to generate poly(ester amide)s with excellent film- and fiber forming properties.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and characterization of a new degradable poly (ester amide) derived from 6-amino-1-hexanol and glutaric acid.
Vera M, et al.
Macromolecules, 36(26), 9784-9796 (2003)
Synthesis of cyclic amines and their alkyl derivatives from amino alcohols over supported copper catalysts.
Kijenski J, et al.
Applied Catalysis, 53(1), 107-115 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service