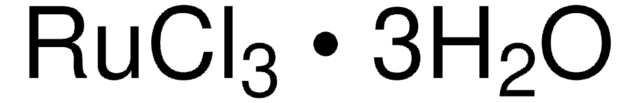931578
Ruthenium(III) chloride hydrate
≥99.9% trace metals basis
Synonym(s):
Ruthenium trichloride, Trichlororuthenium hydrate
About This Item
ethanol: soluble
water: soluble
Recommended Products
grade
for analytical purposes
Quality Level
Assay
≥99.9% trace metals basis
form
powder
impurities
≤1000.0 ppm (trace metals analysis)
solubility
acetone: soluble ((lit.))
ethanol: soluble
water: soluble
density
3.11 g/cm3
SMILES string
O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.H2O.Ru/h3*1H;1H2;/q;;;;+3/p-3
InChI key
BIXNGBXQRRXPLM-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Industrially, ruthenium trichloride hydrate is produced by dissolving ruthenium oxides in hydrochloric acid. The hydrated salt is obtained by recrystallization.
Application
Another common application of ruthenium chloride hydrate is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride hydrate for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
For use in all these applications, also consider our higher-purity ruthenium chloride hydrate, 463779, with trace metals purity greater than 99.98%, which offers the best reproducibility and purity.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service