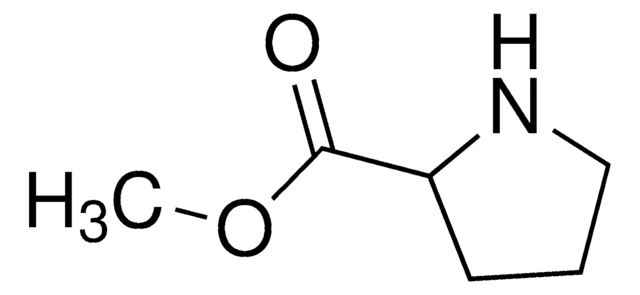
792586
tert-Butyl 3-(2-ethoxy-2-oxoethylidene)azetidine-1-carboxylate
95%
About This Item
Recommended Products
Quality Level
Assay
95%
form
powder
refractive index
n20/D 1.474
density
1.052 g/mL at 25 °C
functional group
ester
storage temp.
2-8°C
SMILES string
CC(C)(C)OC(N(C1)CC1=CC(OCC)=O)=O
InChI
1S/C12H19NO4/c1-5-16-10(14)6-9-7-13(8-9)11(15)17-12(2,3)4/h6H,5,7-8H2,1-4H3
InChI key
WYWJZDFQQJTRDD-UHFFFAOYSA-N
Application
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Spirocyclic modules containing four-membered rings are currently of growing interest to discovery chemists.
Related Content
The Carreira Research Group is focused on expanding and creating access to uncharted landscape in chemical space. In joint efforts with SpiroChem, Carreira develops innovative spirocyclic building blocks, seeking to make them available to the community at large. Molecules constructed from these building blocks take on unique three-dimensional profiles due to the underlying spirocyclic scaffold, enriched by the presence of diverse combinations of exit vectors as sites for functionalization. Importantly, the spirocyclic building blocks possess physicochemical properties useful in the drug discovery process. Thus, drug leads can be tuned through appending these subunits to the periphery of a given scaffold. Moreover, these compact modules represent a useful collection of unprecedented inputs for fragment-based libraries. In all applications, the inherent novelty of the structure affords researchers new opportunities to run wild in their designs and avenues to chemical space – with their imagination as the sole limitations. We are proud to partner in the efforts to make these building blocks widely available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service