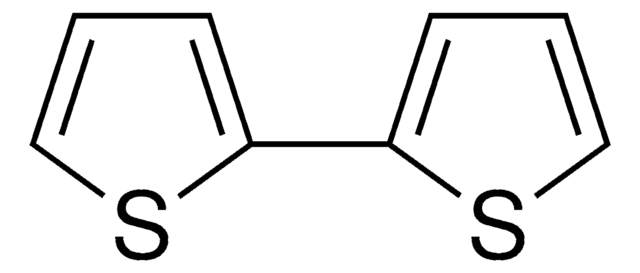
702668
Thieno[3,2-b]thiophene
95%
Synonym(s):
1,4-Dithiapentalene, 1,4-Thiophthene
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
mp
53-58 °C
SMILES string
c1cc2sccc2s1
InChI
1S/C6H4S2/c1-3-7-6-2-4-8-5(1)6/h1-4H
InChI key
VJYJJHQEVLEOFL-UHFFFAOYSA-N
General description
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
210.0 °F
Flash Point(C)
98.9 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The soaring global demand for energy, coupled with the limited supply of fossil fuels, has increased the need for renewable, low-cost energy sources. Organic electronics have shown great promise for applications in lighting, power, and circuitry, with rapidly improving performance already surpassing that of amorphous silicon-based counterparts.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2,5-Dibromothieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/235/282/6821d888-68f9-4334-87b6-abf4792e4651/640/6821d888-68f9-4334-87b6-abf4792e4651.png)
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)
![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
![Dithieno[3,2-b:2′,3′-d]thiophene 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/502/826/9222eb9f-669e-4f11-ad3a-91a3d43058cd/640/9222eb9f-669e-4f11-ad3a-91a3d43058cd.png)
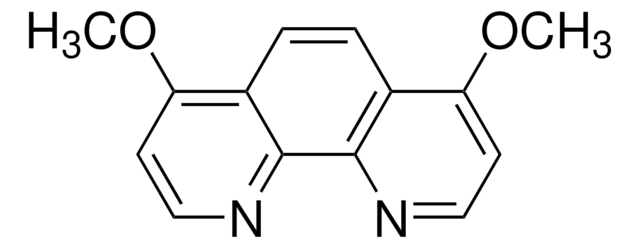
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)
![Thieno[2,3-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/338/511/a37343cf-31cb-4a34-b903-23b3dc8e64ab/640/a37343cf-31cb-4a34-b903-23b3dc8e64ab.png)
![2,6-Dibromodithieno[3,2-b:2′,3′-d]thiophene ≥97%](/deepweb/assets/sigmaaldrich/product/structures/287/437/cf540b93-ec8c-4d2a-897c-dea0a28a8def/640/cf540b93-ec8c-4d2a-897c-dea0a28a8def.png)