565032
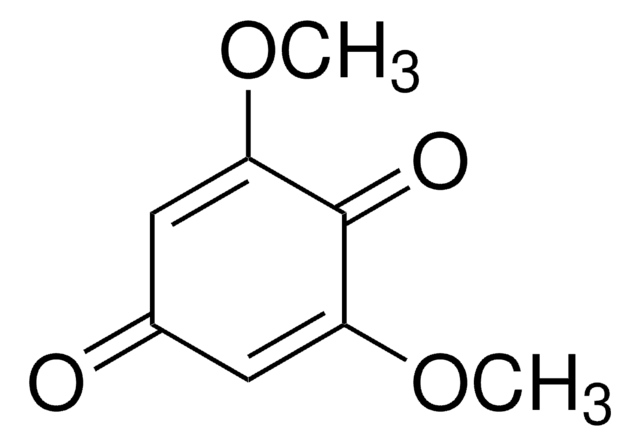
1,4-Dihydroxy-2,6-dimethoxybenzene
97%
Synonym(s):
2,6-Dimethoxyhydroxyquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)2C6H2(OH)2
CAS Number:
Molecular Weight:
170.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
158-162 °C (lit.)
SMILES string
COc1cc(O)cc(OC)c1O
InChI
1S/C8H10O4/c1-11-6-3-5(9)4-7(12-2)8(6)10/h3-4,9-10H,1-2H3
InChI key
GXAVBFNRWXCOPY-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Manickam Selvaraj et al.
Dalton transactions (Cambridge, England : 2003), 48(10), 3291-3299 (2019-02-20)
The major product, 2,3,5-trimethyl-1,4-benzophenone (TMB[double bond, length as m-dash]O), was synthesised by an eco-friendly liquid-phase oxidation of 2,3,6-trimethylphenol (TMP-OH) over Cu(ii)-containing propylsalicylaldimine (CSA) functionalized mesoporous solid catalysts, namely, CSASBA-15(0.2), CSASBA-15(0.1) and CSAMCM-41(0.2), synthesized using various amounts of copper in a
Firoz Shah et al.
Applied and environmental microbiology, 81(24), 8427-8433 (2015-10-04)
Ectomycorrhizal fungi play a key role in mobilizing nutrients embedded in recalcitrant organic matter complexes, thereby increasing nutrient accessibility to the host plant. Recent studies have shown that during the assimilation of nutrients, the ectomycorrhizal fungus Paxillus involutus decomposes organic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service