All Photos(1)
About This Item
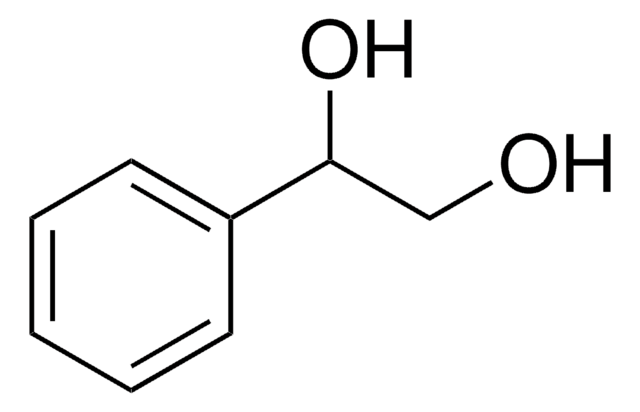
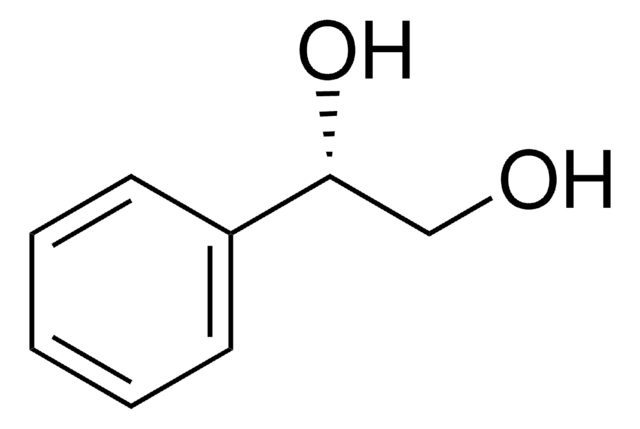
Linear Formula:
HOCH2CH(C6H5)CH2OH
CAS Number:
Molecular Weight:
152.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
53-56 °C (lit.)
SMILES string
OCC(CO)c1ccccc1
InChI
1S/C9H12O2/c10-6-9(7-11)8-4-2-1-3-5-8/h1-5,9-11H,6-7H2
InChI key
BPBDZXFJDMJLIB-UHFFFAOYSA-N
Application
2-Phenyl-1,3-propanediol may be used to synthesize enantiomers of 2-phenyl-3-hydroxypropylcarbamate, via a chemoenzymatic method. It may also be employed in the preparation of 2-phenyl-1,3-propanedithiol.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, characterization and electrochemistry of phenyl-functionalized diiron propanedithiolate complexes.
Li CG, et al.
Polyhedron, 67, 416-421 (2014)
Ryoji Mitsui et al.
Bioscience, biotechnology, and biochemistry, 71(8), 1858-1864 (2007-08-11)
Bacillus cereus 809A and Burkholderia sp. 711C were isolated from soil. These strains demonstrate hydrolysis activity towards prochiral 2-phenyl-1,3-propanediol diacetate and accumulated the corresponding chiral monoacetates into the reaction mixture. When 2-phenyl 1,3-propanediol diacetate was used as a substrate, the
A chemoenzymatic synthesis of both enantiomers of 2-phenyl-3-hydroxypropylcarbamate, a metabolite of felbamate.
Morgan B, et al.
Tetrahedron Asymmetry, 6(7), 1765-1772 (1995)
J Ríha et al.
Veterinarni medicina, 36(2), 65-69 (1991-02-01)
In practical farming conditions of an agricultural enterprise situated in South-East Moravia, the repellents N,N'-diethyl-m-toluamide and 2-phenyl propanediol 1,3 were tested after their application to grazing first-calves of the Bohemian Pied breed. The experiment was conducted in July and August
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service