55624
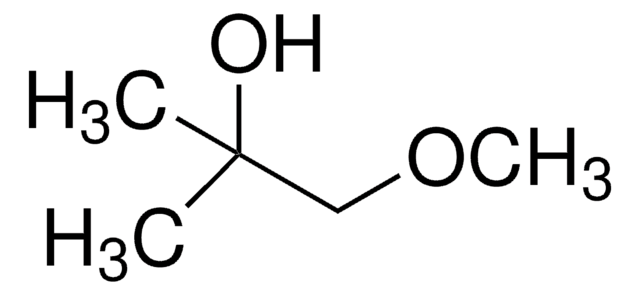
3-Hydroxy-3-methylbutyronitrile
≥97.0% (GC)
Synonym(s):
β-Hydroxyisovaleronitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC(CH3)2CH2CN
CAS Number:
Molecular Weight:
99.13
Beilstein:
1738209
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (GC)
refractive index
n20/D 1.430
bp
114-116 °C/30 mmHg (lit.)
density
0.959 g/mL at 20 °C (lit.)
functional group
hydroxyl
nitrile
storage temp.
2-8°C
SMILES string
CC(C)(O)CC#N
InChI
1S/C5H9NO/c1-5(2,7)3-4-6/h7H,3H2,1-2H3
InChI key
CWPMDJFBWQJRGT-UHFFFAOYSA-N
General description
3-Hydroxy-3-methylbutyronitrile is a β-hydroxynitrile. It undergoes thermal degradation in gas phase via a six-membered cyclic transition state. 3-Hydroxy-3-methylbutyronitrile can be synthesized from 2-hydroxy-2-methyl-1-bromopropane.
Application
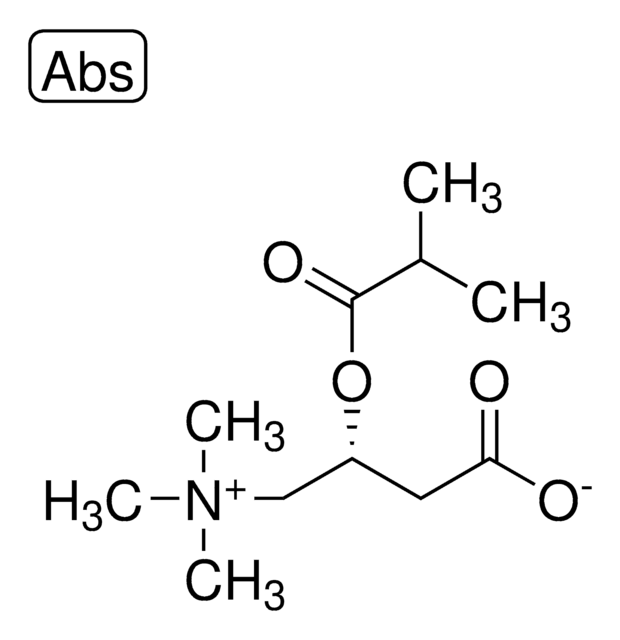
3-Hydroxy-3-methylbutyronitrile may be used to synthesize 1,1-dimethylcyanoethyl bromoacetate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Elimination kinetics of β-hydroxynitriles in the gas phase.
Chuchani G, et al.
Journal of Physical Organic Chemistry, 12, 19-23 (1999)
Douglas J Dellinger et al.
Journal of the American Chemical Society, 125(4), 940-950 (2003-01-23)
Phosphonoacetate and thiophosphonoacetate oligodeoxynucleotides were prepared via a solid-phase synthesis strategy. Under Reformatsky reaction conditions, novel esterified acetic acid phosphinodiamidites were synthesized and condensed with appropriately protected 5'-O-(4, 4'-dimethoxytrityl)-2'-deoxynucleosides to yield 3'-O-phosphinoamidite reactive monomers. These synthons when activated with tetrazole
Theoretical study of the thermolysis reaction of β-hydroxynitriles in the gas phase.
Chamorro E, et al.
International Journal of Quantum Chemistry, 91(5), 618-625 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service