517135
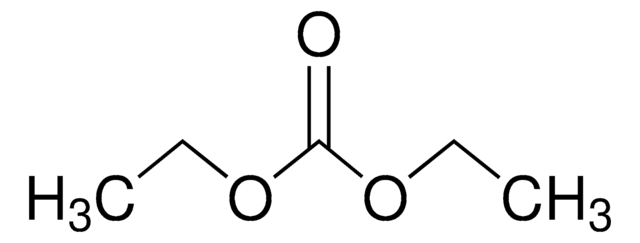
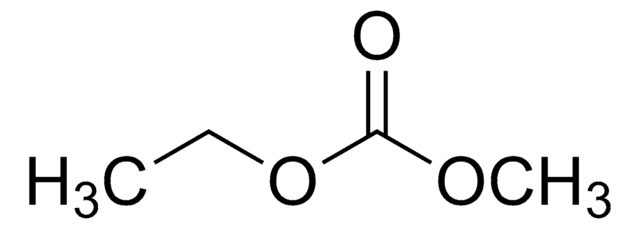
Diethyl carbonate
anhydrous, ≥99%
Synonym(s):
Diatol, Eufin, H-DEC
About This Item
Recommended Products
grade
anhydrous
vapor density
4.1 (vs air)
vapor pressure
10 mmHg ( 23.8 °C)
59 mmHg ( 37.8 °C)
Assay
≥99%
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
<0.002% water
<0.005% water (100 mL)
refractive index
n20/D 1.384 (lit.)
bp
126-128 °C (lit.)
mp
−43 °C (lit.)
solubility
water: insoluble
density
0.975 g/mL at 25 °C (lit.)
greener alternative category
SMILES string
O=C(OCC)OCC
InChI
1S/C5H10O3/c1-3-7-5(6)8-4-2/h3-4H2,1-2H3
InChI key
OIFBSDVPJOWBCH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis of β-enamino esters.
- Synthesis of carbamates and unsymmetrical alkyl carbonates, via reaction with aliphatic amines or alcohols by using a hybrid organic-inorganic material prepared by anchoring TBD to MCM-41 silica.
- As solvent in ruthenium catalyzed direct functionalisation of arene C-H bonds by aryl halides.
- To compose the commercial liquid electrolyte for lithium ion batteries.
- Homogeneous alkoxycarbonylation of cellulose.
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
77.0 °F - closed cup
Flash Point(C)
25 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service