All Photos(1)
About This Item
Linear Formula:
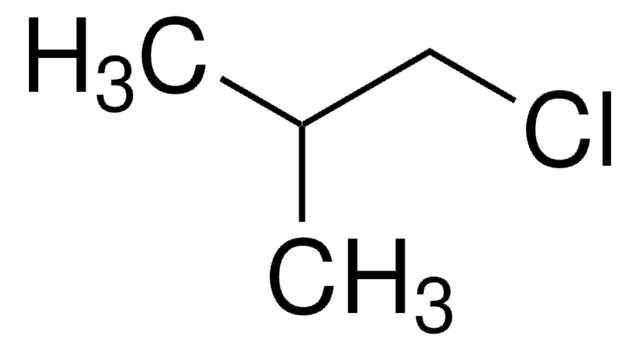
C2H5CH(CH3)CH2Cl
CAS Number:
Molecular Weight:
106.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.412 (lit.)
bp
100 °C/750 mmHg (lit.)
density
0.886 g/mL at 25 °C (lit.)
functional group
alkyl halide
chloro
SMILES string
CCC(C)CCl
InChI
1S/C5H11Cl/c1-3-5(2)4-6/h5H,3-4H2,1-2H3
InChI key
IWAKWOFEHSYKSI-UHFFFAOYSA-N
General description
1-Chloro-2-methylbutane is a halogenated organic building block. It can be synthesized by employing 2-methylbutanol as starting reagent. Grignard reagent derived from (+)-1-chloro-2-methylbutane may be employed for the preparation of partially optically active (-)-alkylphenylcarbinols and partially optically active secondary carbinols. Infrared and Raman spectra of its optically active form (+)(S)-1-chloro-2-methylbutane in various phases (liquid, glass and crystal) have been evaluated.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
32.0 °F - closed cup
Flash Point(C)
0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Infrared and Raman spectra and normal frequencies calculation of isomeric (+)(S)-1-chloro-2-methylbutane.
Schettino V and Benedetti E.
Spectrochimica Acta Part A: Molecular Spectroscopy, 34(3), 353-356 (1978)
Asymmetric Reductions. VII. the Action of the Grignard Reagent from (+)-1-chloro-2-methylbutane on a Series of Alkyl Phenyl Ketones1-3.
MacLeod R, et al.
Journal of the American Chemical Society, 82(4), 876-880 (1960)
Synthetic inhibitors of Alcohol dehydrogenase. 4-Substituted alkyl and cycloalkylpyrazoles.
Tolf BR, et al.
Acta Chemica Scandinavica, 33, 483-487 (1979)
Asymmetric Reductions. VI. The Action of the Grignard Reagent from (+)-1-Chloro-2-methylbutane on a Series of Alkyl t-Butyl Ketones1.
Foley WM, et al.
Journal of the American Chemical Society, 81(11), 2779-2784 (1959)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service