47311
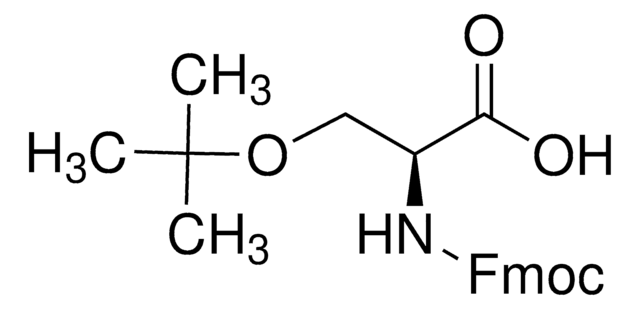
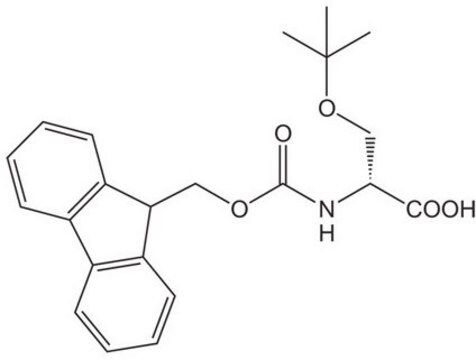
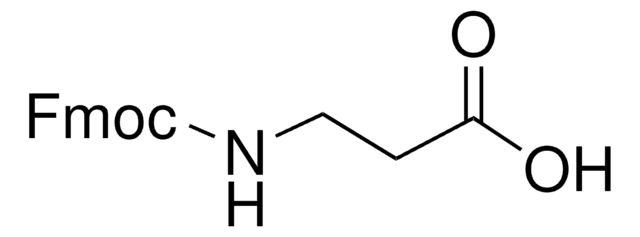
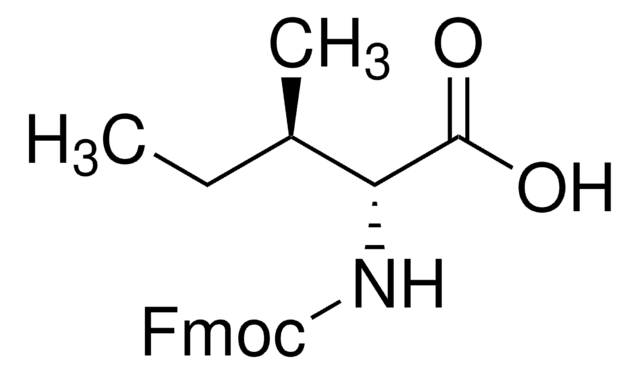
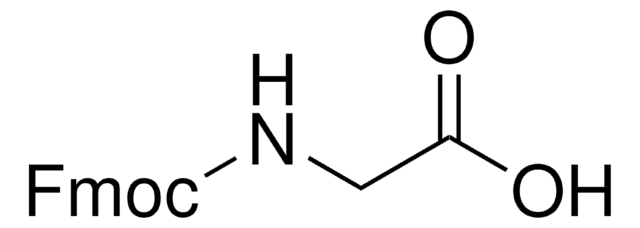
Fmoc-D-Ser(tBu)-OH
≥98.0% (TLC)
Synonym(s):
Fmoc-O-tert-butyl-D-serine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H25NO5
CAS Number:
Molecular Weight:
383.44
Beilstein:
5309984
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥98.0% (TLC)
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
CC(C)(C)OC[C@@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C22H25NO5/c1-22(2,3)28-13-19(20(24)25)23-21(26)27-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,23,26)(H,24,25)/t19-/m1/s1
InChI key
REITVGIIZHFVGU-LJQANCHMSA-N
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fayçal Touti et al.
Nature chemical biology, 15(4), 410-418 (2019-03-20)
The use of competitive inhibitors to disrupt protein-protein interactions (PPIs) holds great promise for the treatment of disease. However, the discovery of high-affinity inhibitors can be a challenge. Here we report a platform for improving the affinity of peptide-based PPI
Andreas Pech et al.
Nucleic acids research, 45(7), 3997-4005 (2017-02-06)
Biological evolution resulted in a homochiral world in which nucleic acids consist exclusively of d-nucleotides and proteins made by ribosomal translation of l-amino acids. From the perspective of synthetic biology, however, particularly anabolic enzymes that could build the mirror-image counterparts
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service