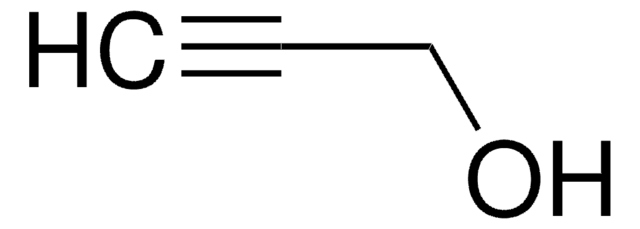448729
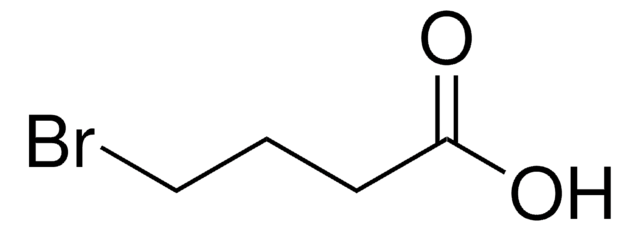
9-Bromo-1-nonanol
95%
Synonym(s):
Nonamethylene bromohydrin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)9OH
CAS Number:
Molecular Weight:
223.15
Beilstein:
1737525
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
125-126 °C/2 mmHg (lit.)
mp
33-35 °C (lit.)
SMILES string
OCCCCCCCCCBr
InChI
1S/C9H19BrO/c10-8-6-4-2-1-3-5-7-9-11/h11H,1-9H2
InChI key
USJDOLXCPFASNV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sequential alkynylation of ω-bromoalkyl triflates: facile access to unsymmetrical non-conjugated diynes including precursors to diene.
Armstrong-Chong RJ, et al.
Tetrahedron, 60(45), 10239-10244 (2004)
Synthesis of the diacetylenic phospholipids 1, 2-(10', 12'-heptadecadiynoyl)-sn-glycero-3-phophatidylcholine and 1, 2-(4', 6'-tricosadiynoyl)-sn-glycero-3-phophatidylcholine.
Hennies PT, et al.
Journal of the Brazilian Chemical Society, 12(1), 64-72 (2001)
In vitro enzymatic oxidation of a fluorine-tagged sulfido substrate analogue: a 19F NMR investigation.
Tremblay AE, et al.
Magnetic Resonance in Chemistry, 44(6), 629-632 (2006)
Néstor M Carballeira et al.
Chemistry and physics of lipids, 145(1), 37-44 (2006-11-28)
The first total syntheses for the (Z)-15-methyl-10-hexadecenoic acid and the (Z)-13-methyl-8-tetradecenoic acid were accomplished in seven steps and in 31-32% overall yields. The (trimethylsilyl)acetylene was the key reagent in both syntheses. It is proposed that the best synthetic strategy towards
Highly ion conductive flexible films composed of network polymers based on polymerizable ionic liquids.
Washiro S, et al.
Polymer, 45(5), 1577-1582 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Poly[4,4′-methylenebis(phenyl isocyanate)-alt-1,4-butanediol/di(propylene glycol)/polycaprolactone] pellets, MDI-polyester/polyether polyurethane.](/deepweb/assets/sigmaaldrich/product/structures/661/697/b23c24ce-15fb-4eae-a30f-786921d4c91e/640/b23c24ce-15fb-4eae-a30f-786921d4c91e.png)