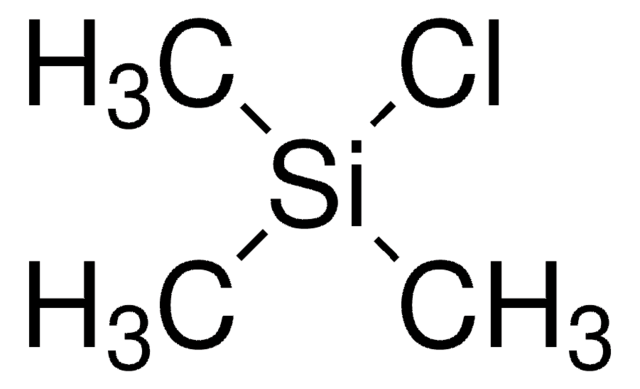440442
O-(Trimethylsilyl)hydroxylamine
technical grade, 90%
Synonym(s):
Aminoxytrimethylsilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
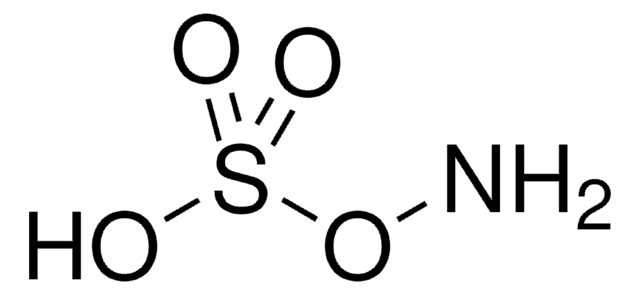
Linear Formula:
(CH3)3SiONH2
CAS Number:
Molecular Weight:
105.21
Beilstein:
2035893
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
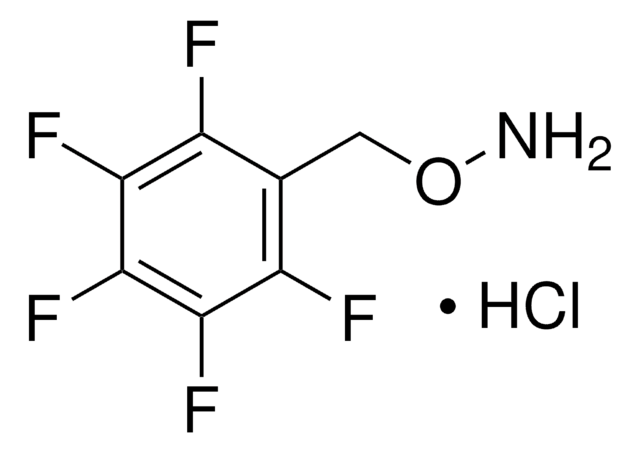
Recommended Products
grade
technical grade
Assay
90%
form
liquid
refractive index
n20/D 1.405 (lit.)
bp
98-100 °C (lit.)
density
0.86 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)ON
InChI
1S/C3H11NOSi/c1-6(2,3)5-4/h4H2,1-3H3
InChI key
AEKHNNJSMVVESS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
O-(Trimethylsilyl)hydroxylamine is an O-substituted hydroxylamine. It is formed as an intermediate during the trimethylsilyl chloride catalyzed synthesis of α-branched amines.
Application
O-(Trimethylsilyl)hydroxylamine may be used for the preparation of O-trimethylsilyl oxime ethers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
53.6 °F - closed cup
Flash Point(C)
12 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ying Fu et al.
Organic & biomolecular chemistry, 10(38), 7669-7672 (2012-08-14)
A general procedure for the nucleophilic addition of organozinc halides with nitrones in the presence of trimethylsilyl chloride has been developed. Trimethylsilyl chloride was found to be both an indispensable reaction promoter and a ready hydroxylamine protective agent in these
Chemoselective addition of organometallics to oxime ethers.
Tavakol H,et al.
Journal of Organometallic Chemistry, 692(10), 1924-1927 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service