409022
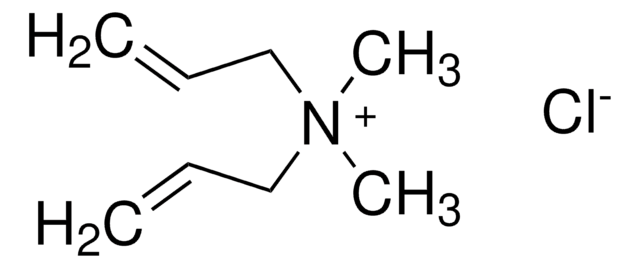
Poly(diallyldimethylammonium chloride) solution
average Mw 200,000-350,000 (medium molecular weight), 20 wt. % in H2O
Synonym(s):
PDADMAC
About This Item
Recommended Products
form
viscous liquid
mol wt
average Mw 200,000-350,000 (medium molecular weight)
concentration
20 wt. % in H2O
refractive index
n20/D 1.375
viscosity
250-500 cP(25 °C, Brookfield)
density
1.04 g/mL at 25 °C
InChI
1S/C8H16N.ClH/c1-5-7-9(3,4)8-6-2;/h5-6H,1-2,7-8H2,3-4H3;1H/q+1;/p-1
InChI key
GQOKIYDTHHZSCJ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article that discusses two applications in particular; first, using these layers as polyelectrolyte membranes to control permeability.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Poly[bis(2-chloroethyl) ether-alt-1,3-bis[3-(dimethylamino)propyl]urea] quaternized, solution 62 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/139/843/f0b4a2ac-de44-4fb5-ab36-a700daaf8aa5/640/f0b4a2ac-de44-4fb5-ab36-a700daaf8aa5.png)