All Photos(2)
About This Item
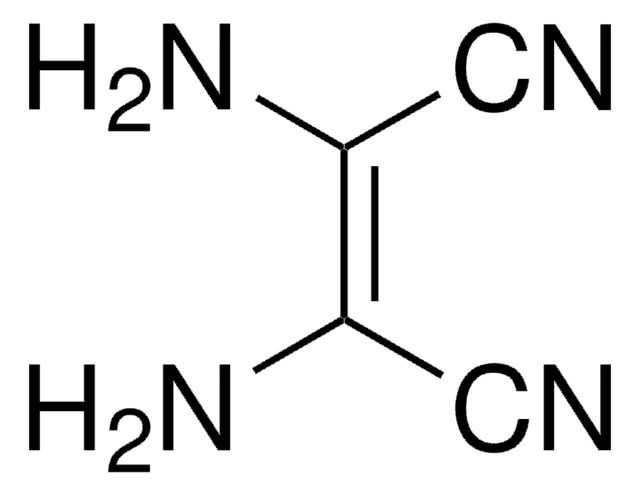
Linear Formula:
C2H5OC(=NH)CH2C(=NH)OC2H5·2HCl
CAS Number:
Molecular Weight:
231.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
impurities
<5% ammonium chloride
mp
122 °C (dec.) (lit.)
functional group
amine
ether
SMILES string
Cl.Cl.CCOC(=N)CC(=N)OCC
InChI
1S/C7H14N2O2.2ClH/c1-3-10-6(8)5-7(9)11-4-2;;/h8-9H,3-5H2,1-2H3;2*1H
InChI key
TWUQXVGHXWRUBR-UHFFFAOYSA-N
Application
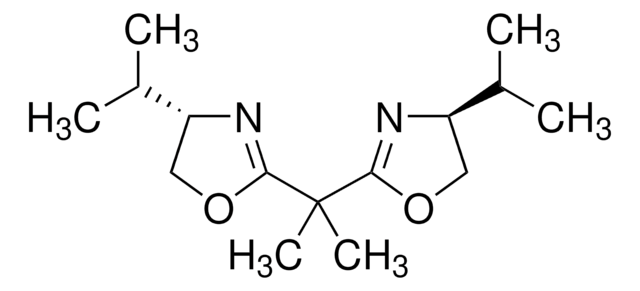
Diethyl malonimidate dihydrochloride may be used in the preparation of chiral bis(oxazoline) ligands. It may be used in the preparation of intramolecularly cross-linked urokinase.
Reactant for:
- Preparation of rhenium cyanobis(oxazoline) oxo complexes as enantioselective reduction catalysts
- Synthesis of chiral bis(oxazoline)-copper complexes immobilized by donor-acceptor interactions on insoluble organic supports as reusable catalysts for asymmetric Diels-Alder cycloaddition
- Preparation of nonracemic bis(oxazoline)ruthenium p-cymene complexes and silica-supported analogs as catalysts for the enantioselective transfer hydrogenation of ketones to secondary alcohols
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemical modification of urokinase with bis-imidoesters and properties of the intramolecularly cross-linked enzyme.
Yokoigawa K, et al.
Agricultural and Biological Chemistry, 53(7), 1837-1842 (1989)
New chiral bis (oxazoline) Rh (I)-, Ir (I)-and Ru (II)-complexes for asymmetric transfer hydrogenations of ketones.
Debono N, et al.
Tetrahedron Letters, 45(10), 2235-2238 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![(+)-2,2′-Isopropylidenebis[(4R)-4-phenyl-2-oxazoline] 97%](/deepweb/assets/sigmaaldrich/product/structures/232/241/07f8baaa-0ba2-49e0-8ac2-f6d256fb2c84/640/07f8baaa-0ba2-49e0-8ac2-f6d256fb2c84.png)