391549
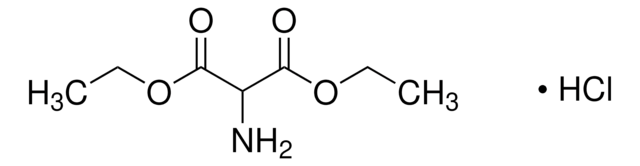
Dimethyl aminomalonate hydrochloride
97%
Synonym(s):
Aminomalonic acid dimethyl ester hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH(COOCH3)2 · HCl
CAS Number:
Molecular Weight:
183.59
Beilstein:
3696467
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
mp
160 °C (dec.) (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless to faintly yellow
SMILES string
Cl[H].COC(=O)C(N)C(=O)OC
InChI
1S/C5H9NO4.ClH/c1-9-4(7)3(6)5(8)10-2;/h3H,6H2,1-2H3;1H
InChI key
QWNDKNJSEWOEDM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Dimethyl aminomalonate hydrochloride is a hydrochloride salt of a dialkyl aminomalonate.
Application
Dimethyl aminomalonate hydrochloride (aminomalonic acid dimethyl ester hydrochloride) may be used in synthesis of methyl 3-phenyl-5-hydantoincarboxylate and Boc-Leu-Ama(OMe)2(Boc= tert-butyloxycarbonyl, Leu= leucine, Ama= aminomalonic acid). It may be used as starting reagent in the synthesis of the following:
- (R,S)-2-phenethylcysteine hydrochloride
- dimethyl 2,2,2-polynitroalkylnitroaminonitromalonate
- spirotryprostatin B
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of Nitro Compounds Starting with Dialkyl Aminomalonates.
Ishchenko MA, et al.
Russ. J. Org. Chem., 37(2), 194-197 (2001)
D Krumme et al.
FEBS letters, 436(2), 209-212 (1998-10-22)
Novel peptides containing the sequence -Pro-Leu-Ama(NHOH)- were synthesized and characterized by spectroscopic techniques. Their inhibitory properties towards the activated form of native human gelatinase B (MMP-9) and the catalytic domain of neutrophil collagenase (cdMMP-8) were determined. The most effective inhibitor
Alpha-alkylcysteines as inhibitors for carboxypeptidase A. Synthesis, evaluation, and implication for inhibitor design strategy.
Lee HS and Kim DH.
Bull. Korean Chem. Soc., 23(4), 593-598 (2002)
Heterocyclizations, XIV. 1,3,5,7,-Tetraoxoperhydroimidazo[1,5-c]imidazole, a Novel Bridgehead Nitrogen Ureide
Capuano L, et al.
Chemische Berichte, 107(10), 3237-3245 (1974)
Oxindole as starting material in organic synthesis.
Ziarani GM, et al.
ARKIVOC (Gainesville, FL, United States), 1, 470-535 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service