343668
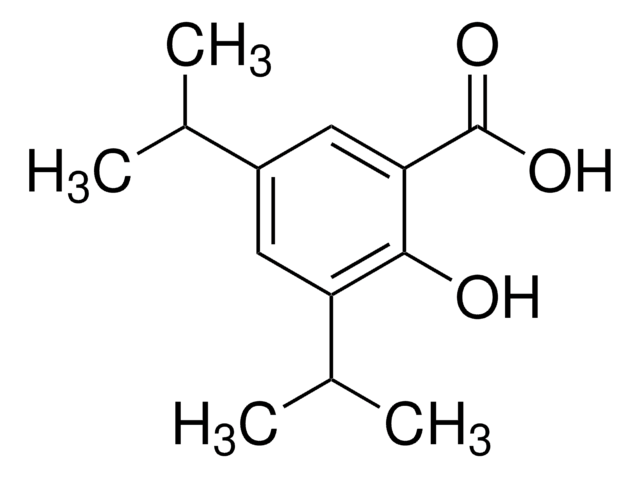
2-Hydroxy-3-isopropylbenzoic acid
98%
Synonym(s):
3-Isopropylsalicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHC6H3(OH)CO2H
CAS Number:
Molecular Weight:
180.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
73-75 °C (lit.)
functional group
carboxylic acid
SMILES string
CC(C)c1cccc(C(O)=O)c1O
InChI
1S/C10H12O3/c1-6(2)7-4-3-5-8(9(7)11)10(12)13/h3-6,11H,1-2H3,(H,12,13)
InChI key
XGAYQDWZIPRBPF-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554)
Related Categories
General description
2-Hydroxy-3-isopropylbenzoic acid is an analog of general anesthetic compound, propofol (2,6-diisopropylphenol). It was investigated for general anesthetic activity in Xenopus laevis tadpoles and for the ability to produce enhancement of submaximal GABA responses.
Application
2-Hydroxy-3-isopropylbenzoic acid may be used in the preparation of 3-isopropylgentisic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M D Krasowski et al.
The Journal of pharmacology and experimental therapeutics, 297(1), 338-351 (2001-03-22)
A series of 27 analogs of the general anesthetic propofol (2,6-diisopropylphenol) were examined for general anesthetic activity in Xenopus laevis tadpoles and for the ability to produce enhancement of submaximal GABA responses and/or direct activation at recombinant GABA(A) receptors. Fourteen
D J Hopper et al.
The Biochemical journal, 122(1), 29-40 (1971-03-01)
1. Cell-free extracts, prepared from a non-fluorescent Pseudomonas grown on m-cresol, oxidized gentisate and certain alkyl-substituted gentisates with the consumption of 1 mol of oxygen and the formation of 1 mol of pyruvate from 1 mol of substrate. 2. In
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service