283061
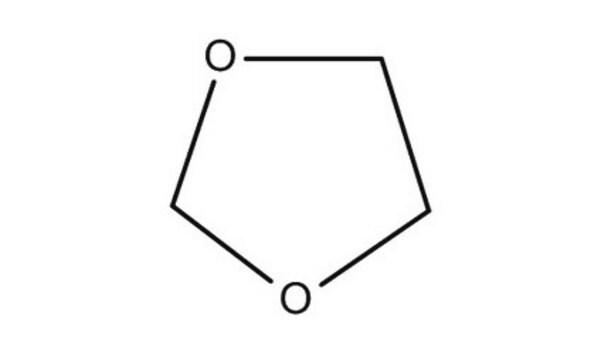
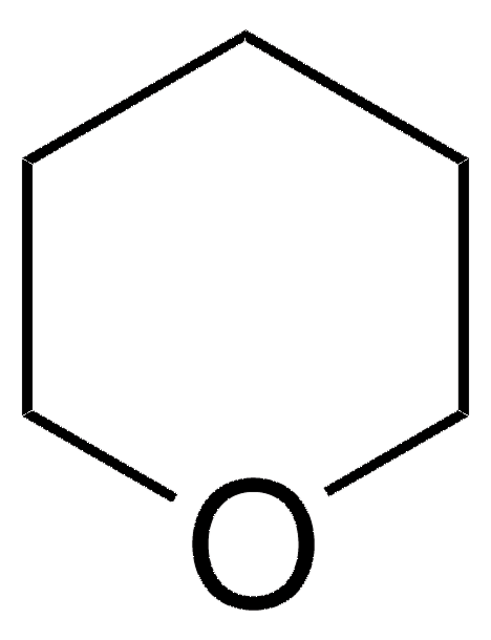
1,3-Dioxane
97%
Synonym(s):
Formaldehyde trimethylene acetal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O2
CAS Number:
Molecular Weight:
88.11
Beilstein:
102532
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
impurities
<3% 1,3,5-trioxane
refractive index
n20/D 1.418 (lit.)
bp
105-106 °C (lit.)
mp
−45 °C (lit.)
density
1.032 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
C1COCOC1
InChI
1S/C4H8O2/c1-2-5-4-6-3-1/h1-4H2
InChI key
VDFVNEFVBPFDSB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The reactivity of molybdenum pentachloride with1,3-dioxane has been investigated at room temperature in a non-coordinating solvent (dichloromethane).
Application
1,3-Dioxane has been employed as solvent in the synthesis of 8- and 9-membered dioxazocines and dioxazonines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
41.0 °F - closed cup
Flash Point(C)
5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Florian Medina et al.
Organic letters, 16(12), 3232-3235 (2014-06-03)
Eight- and 9-membered dioxazocines and dioxazonines are readily synthesized starting from N-sulfonyl-1,2,3-triazoles in a single-step procedure. A perfect regioselectivity and generally good yields (up to 92%) are obtained under dirhodium catalysis using 1,3-dioxolane and 1,3-dioxane as solvents and reagents.
Fabio Marchetti et al.
Dalton transactions (Cambridge, England : 2003), 42(42), 15226-15234 (2013-09-06)
The reactivity of molybdenum pentachloride, 1, with a selection of mono- and diethers was investigated at room temperature in a non-coordinating solvent (dichloromethane). The Mo(IV) complex MoCl4(OMe2)2, 2, was obtained in 75% yield by the reaction of 1 with an
Mei Sun et al.
Environmental science & technology, 50(5), 2246-2254 (2016-02-02)
Recent U.S. Environmental Protection Agency data show that 1,4-dioxane is frequently detected in U.S. drinking water derived from both groundwater and surface water. 1,4-Dioxane is a likely human carcinogen, and an excess 10(-6) cancer risk is associated with a drinking
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 283061-25G | |
| 283061-5G | 4061826236659 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service