258601
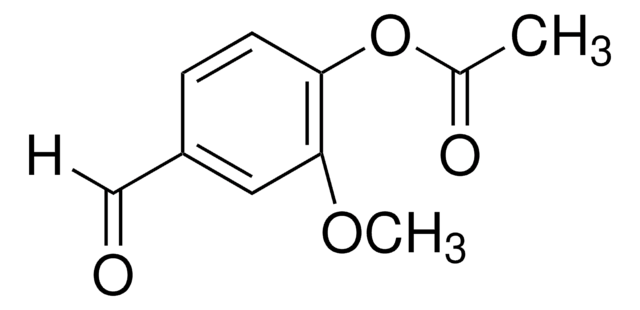
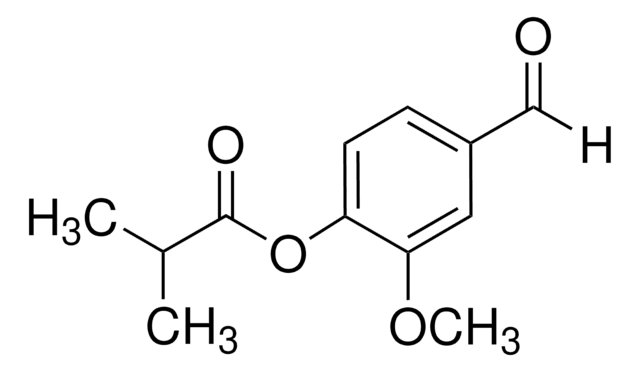
Vanillin acetate
98%
Synonym(s):
4-Formyl-2-methoxyphenyl acetate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CO2C6H3-4-(CHO)-2-OCH3
CAS Number:
Molecular Weight:
194.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
77-79 °C (lit.)
functional group
aldehyde
ester
SMILES string
COc1cc(C=O)ccc1OC(C)=O
InChI
1S/C10H10O4/c1-7(12)14-9-4-3-8(6-11)5-10(9)13-2/h3-6H,1-2H3
InChI key
PZSJOBKRSVRODF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Chemoselective reduction of vanillin acetate using sodium borohydride has been reported.
Application
Vanillin acetate has been used in preparation of:
- 2-nitrohomovanillic acid
- 2-nitrovanildin acetate
- 2-nitro-3,4-duimethoxybenzaldehyde
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Azlactones and phenylacetic acids derived from the 2-nitro-derivatives of vanillin, isovanillin, and veratraldehyde.
S F MacDONALD
Journal of the Chemical Society, 174, 376-378 (1948-03-01)
The discovery-oriented approach to organic chemistry. 6. Selective reduction in organic chemistry: Reduction of aldehydes in the presence of esters using sodium borohydride.
Baru AR and Mohan RS
Journal of Chemical Education, 82(11), 1674-1674 (2005)
New synthesis of 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone and hexahydrocoenzyme Q4 chromanol.
Weinstock LM, et al.
Journal of Chemical and Engineering Data, 12(1), 154-155 (1967)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service