246174
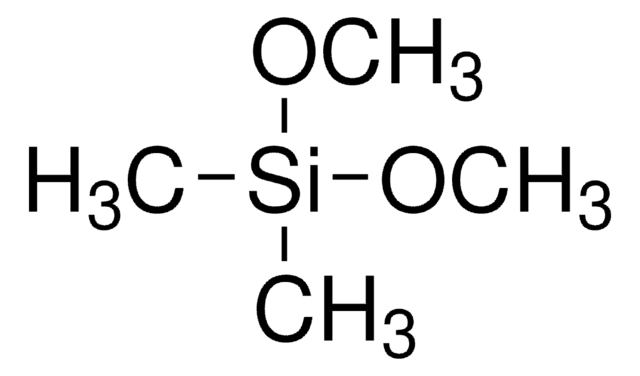
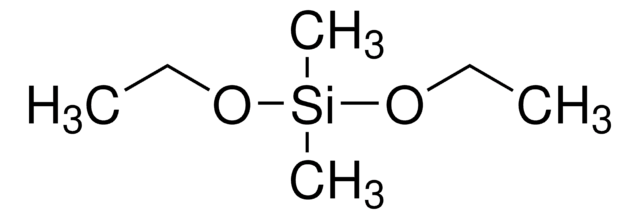
Trimethoxymethylsilane
98%
Synonym(s):
Methyltrimethoxysilane
About This Item
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.371 (lit.)
bp
102-104 °C (lit.)
density
0.955 g/mL at 25 °C (lit.)
SMILES string
CO[Si](C)(OC)OC
InChI
1S/C4H12O3Si/c1-5-8(4,6-2)7-3/h1-4H3
InChI key
BFXIKLCIZHOAAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a silica source for synthesizing polyethyleneimine-silica (PEI-silica) organic-inorganic hybrid particles.
- To transform hydrophilic ceramic surfaces to hydrophobic by modifying the -OH groups.
- To modify silica aerogels by inducing hydrophobicity and enhancing mechanical properties without affecting transparency.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
48.2 °F
Flash Point(C)
9 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service