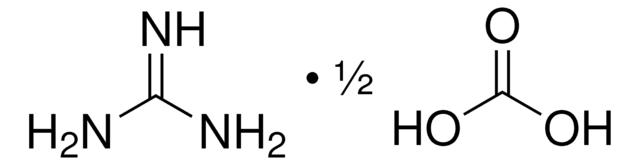234249
Guanidine nitrate
98%
Synonym(s):
Guanidinium nitrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2C(=NH)NH2 · HNO3
CAS Number:
Molecular Weight:
122.08
Beilstein:
3596600
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
solid
Assay:
98%
Recommended Products
Quality Level
Assay
98%
form
solid
mp
213-215 °C (lit.)
solubility
water: soluble 50 mg/mL, clear to very slightly hazy, colorless
functional group
amine
nitrate
SMILES string
NC(N)=N.O[N+]([O-])=O
InChI
1S/CH5N3.HNO3/c2*2-1(3)4/h(H5,2,3,4);(H,2,3,4)
InChI key
CNUNWZZSUJPAHX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Guanidine nitrate (GN) is an explosive used by the military and commercial sectors. Decomposition of guanidine nitrate has been investigated.
Application
Guanidine nitrate has been used:
- in ammonium nitrate explosive formulations and in various propellant applications including triple-base gun propellants
- to examine the vapor signatures of the nitrate salts of guanidine by isothermal thermogravimetric method
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Ox. Sol. 3
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yunfei Zhou et al.
Environmental science and pollution research international, 26(10), 9861-9875 (2019-02-09)
Montmorillonite grafted polyacrylic acid composite (GNM) was prepared by using ultraviolet radiation grafting method in this work. The synthesized materials were characterized by XRF, SEM, FTIR, XRD, TG, and XPS. The experimental equilibrium data indicates that the adsorbent is suitable
Decompositions of urea and guanidine nitrates.
Oxley JC, et al.
Journal of Energetic Materials, 27(1), 17-39 (2008)
Determination of urea nitrate and guanidine nitrate vapor pressures by isothermal thermogravimetry.
Oxley J, et al.
Propellants, Explosives, Pyrotechnics, 35(3), 278-283 (2010)
Yeon-Mi Lim et al.
Toxics, 8(3) (2020-09-26)
The toxicity profiles of the widely used guanidine-based chemicals have not been fully elucidated. Herein, we evaluated the in vitro and in vivo toxicity of eight guanidine-based chemicals, focusing on inhalation toxicity. Among the eight chemicals, dodecylguanidine hydrochloride (DGH) was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service