233625
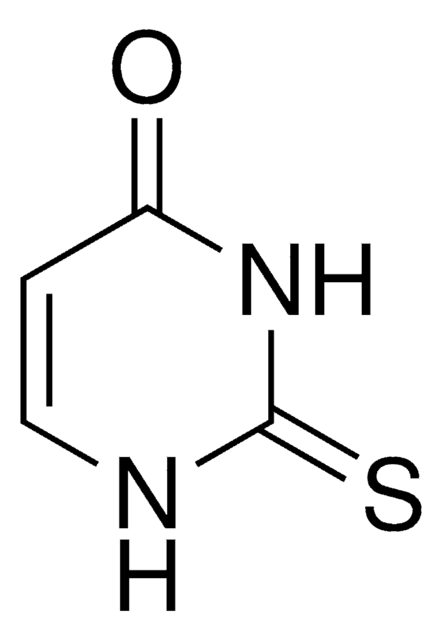
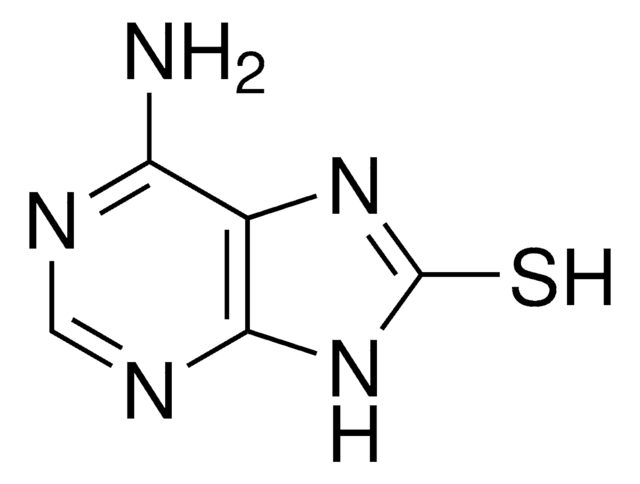
2-Thiocytosine
97%
Synonym(s):
4-Amino-2-mercaptopyrimidine, 4-Amino-2-thiopyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3S
CAS Number:
Molecular Weight:
127.17
Beilstein:
112435
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
285-290 °C (dec.) (lit.)
SMILES string
NC1=NC(=S)NC=C1
InChI
1S/C4H5N3S/c5-3-1-2-6-4(8)7-3/h1-2H,(H3,5,6,7,8)
InChI key
DCPSTSVLRXOYGS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Thiocytosine is a potential antileukemic and anticancer agent. 2-Thiocytosine in solid state has been investigated by (1)H-(14)N NMR-NQR double resonance (NQDR) spectroscopy and theoretically by the quantum theory of atoms in molecules (QTAIM)/density functional theory (DFT).
Application
2-Thiocytosine has been used to investigate the reactions involved in hole transfer (HT) in X-irradiated doped cytosine monohydrate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
André Krivokapić et al.
The journal of physical chemistry. A, 112(16), 3597-3606 (2008-03-18)
Following exposure to X-irradiation at low temperatures, the main reactions taking place in single crystals of cytosine monohydrate doped with minute amounts of 2-thiocytosine are hole transfer (HT) from the electron-loss centers to the dopant and recombination of oxidation and
Sebastian Mai et al.
The journal of physical chemistry. B, 121(20), 5187-5196 (2017-04-30)
The solvatochromic effects of six different solvents on the UV absorption spectrum of 2-thiocytosine have been studied by a combination of experimental and theoretical techniques. The steady-state absorption spectra show significant shifts of the absorption bands, where in more polar
Theoretical and matrix-isolation experimental studies on 2-thiocytosine and 5-fluoro-2-thiocytosine.
H Rostkowska et al.
Biochimica et biophysica acta, 1172(3), 239-246 (1993-03-20)
2-Thiocytosine (s2Cyt) and 5-fluoro-2-thiocytosine (f5s2Cyt) were studied by means of IR spectroscopy under different environmental conditions: isolated in low-temperature inert gas matrices, associated in thin amorphous and polycrystalline films. The compounds isolated in matrices were only very slightly influenced by
Determination of thiocytosine using its enhancement effect on the copper anodic stripping wave.
R B Diez-Caballero et al.
The Analyst, 113(7), 1047-1050 (1988-07-01)
Jolanta N Latosińska et al.
Journal of molecular modeling, 18(1), 11-26 (2011-03-30)
A potential antileukemic and anticancer agent, 2-thiocytosine (2-TC), has been studied experimentally in the solid state by (1)H-(14)N NMR-NQR double resonance (NQDR) and theoretically by the quantum theory of atoms in molecules (QTAIM)/density functional theory (DFT). Eighteen resonance frequencies on
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 233625-5G | |
| 233625-1G | 4061826221662 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service