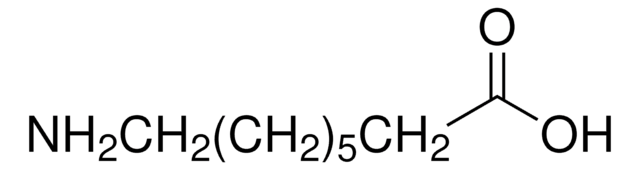217700
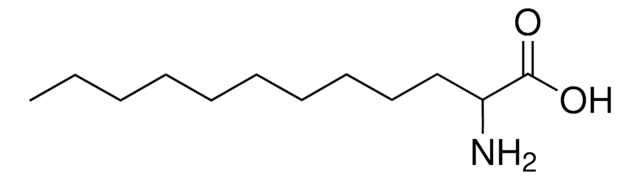
DL-2-Aminocaprylic acid
99%
Synonym(s):
DL-2-Aminooctanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
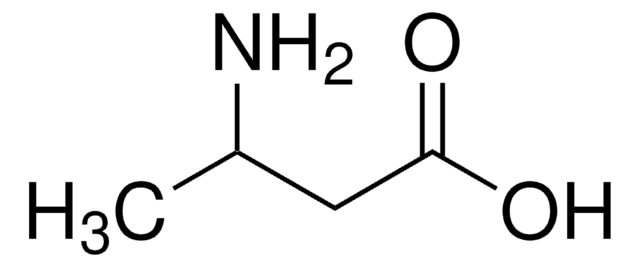
CH3(CH2)5CH(NH2)CO2H
CAS Number:
Molecular Weight:
159.23
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
260 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
CCCCCCC(N)C(O)=O
InChI
1S/C8H17NO2/c1-2-3-4-5-6-7(9)8(10)11/h7H,2-6,9H2,1H3,(H,10,11)
InChI key
AKVBCGQVQXPRLD-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Seon-Hee Kim et al.
PLoS neglected tropical diseases, 6(10), e1868-e1868 (2012-11-15)
Fatty acid (FA) binding proteins (FABPs) of helminths are implicated in acquisition and utilization of host-derived hydrophobic substances, as well as in signaling and cellular interactions. We previously demonstrated that secretory hydrophobic ligand binding proteins (HLBPs) of Taenia solium metacestode
Arik Dahan et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 108, 78-85 (2017-06-20)
The enzyme phospholipase A
Milica Markovic et al.
Pharmaceutics, 11(4) (2019-04-19)
In ulcerative colitis (UC), the inflammation is localized in the colon, and one of the successful strategies for colon-targeting drug delivery is the prodrug approach. In this work, we present a novel phospholipid (PL)-based prodrug approach, as a tool for
Paper chromatography of 56 amino compounds using phenol and butanol-acetic acid as solvents with illustrative chromatograms of normal and abnormal urines.
T E PARRY
Clinica chimica acta; international journal of clinical chemistry, 2(2), 115-125 (1957-04-01)
Sarah A Almahboub et al.
Applied microbiology and biotechnology, 102(2), 789-799 (2017-11-28)
Terminal modification of peptides is frequently used to improve their hydrophobicity. While N-terminal modification with fatty acids (lipidation) has been reported previously, C-terminal lipidation is limited as it requires the use of linkers. Here we report the use of a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service