All Photos(3)
About This Item
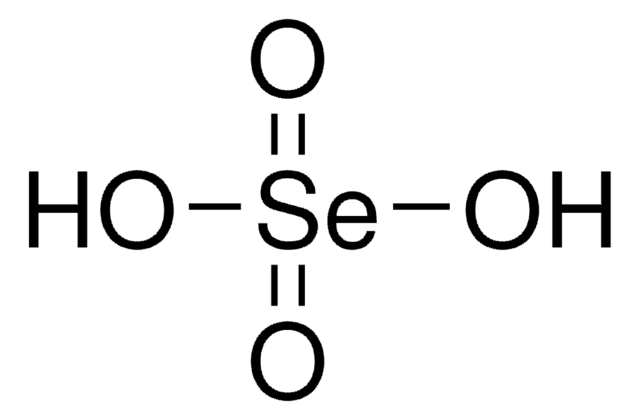
Linear Formula:
H2SeO3
CAS Number:
Molecular Weight:
128.97
EC Number:
MDL number:
UNSPSC Code:
12352301
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
70 °C (dec.) (lit.)
density
3.004 g/mL at 25 °C (lit.)
SMILES string
O[Se](O)=O
InChI
1S/H2O3Se/c1-4(2)3/h(H2,1,2,3)
InChI key
MCAHWIHFGHIESP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Selenous acid can be used as a reagent to synthesize:
- α-Ketoacectals by reacting with acetophenones and triethylorthoformate in the presence of boron trifluoride etherate as a catalyst.
- 4-Formyl-7-methylcoumarin using 4,7-dimethylcoumarin in the presence of xylene as a solvent.
- Selenium substituted quinoxaline derivatives from 2,3-dichloro quionoxaline.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D Seidel et al.
Journal of clinical chemistry and clinical biochemistry. Zeitschrift fur klinische Chemie und klinische Biochemie, 16(7), 407-411 (1978-07-01)
A simple one-vial-method was developed for the quantitative determination of sphingomyelinase activity in human leukocytes and urine, using [14C-methyl] sphingomyelin. The measured activities of healthy control persons show a higher scatter in (n=50) urine (1.2 +/- 0.5 nmol/h . ml
One-pot synthesis of alpha-ketoacetals from aryl methyl ketones in the presence of selenous acid catalyzed by boron trifluoride etherate
Kharkongor I, et al.
Tetrahedron Letters, 56, 4359-4362 (2015)
Thomas G Back et al.
Journal of the American Chemical Society, 124(41), 12104-12105 (2002-10-10)
1,2-Oxaselenolane Se-oxide is a novel cyclic seleninate ester that functions as a remarkably efficient glutathione peroxidase mimetic by catalyzing the reduction of tert-butyl hydroperoxide to tert-butyl alcohol in the presence of benzyl thiol. The seleninate ester can be conveniently generated
Y Kayanoki et al.
Journal of biochemistry, 119(4), 817-822 (1996-04-01)
Selenium-dependent glutathione peroxidase (GPx) plays a protective role in oxidative stress-induced apoptosis. In this study, we demonstrated that MDBK cells, a bovine renal epithelial cell line, exhibited internucleosomal DNA fragmentation characteristic of apoptotic cell death under selenium-deficient conditions with lower
Yuki Ohta et al.
Toxicology and applied pharmacology, 226(2), 169-177 (2007-11-09)
All nutritional selenium sources are transformed into the assumed common intermediate selenide for the syntheses of selenoproteins for utilization and/or of selenosugar for excretion. Methylselenol [monomethylselenide, MMSe] is the assumed intermediate leading to other methylated metabolites, dimethylselenide (DMSe) and trimethylselenonium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service